Aquatic Research Laboratory, Marineland, Moorpark, California 93021
Two bioassay tests were conducted to evaluate the effects of recently mixed commercial synthetic sea salts (SSS) and natural seawater (NSW) on the development of larvae of the White Sea urchin Lytichinus pictus. Freshly fertilized sea urchin eggs were placed in solutions of the different SSS and NSW for 72 h at room temperature after which percent fertilization and larvae development were accessed. The results showed that no SSS performed significantly better or worse than any other SSS in terms of percent normal development of the sea urchin larvae. In one bioassay it was clearly demonstrated that the use of a natural seawater product (Catalina Sea Water) resulted in significantly poorer development of newly fertilized sea urchin larvae. No relationship was found between decreasing percentage of normal sea urchin development and increasing amount of total trace element concentration. The results of these tests show that SSS are not detrimental to newly fertilized sea urchin eggs, and by extension, marine aquaria.
Introduction
A frequent question in the marine aquarium hobby in whether synthetic sea salts are an acceptable substitute for natural seawater for use in marine fish and coral reef aquaria; the assumption being that the natural seawater available to hobbyist is of the best quality. One way to evaluate this question is through the chemical analysis of major, minor and trace elements. However, chemical analyses do not always provide a definitive answer because: 1) the general chemical composition of most SSS mirrors that of NSW, at least in terms of the concentration of many major and minor elements, 2) the analysis and chemistry of trace elements in seawater is difficult to accurately access and 3) certain chemicals and elements could act synergistically and increase, or decrease, the toxicity of the SSS.
Another, non-chemical but biological, way to evaluate and compare SSS to each other and NSW is through the conducting of tests called bioassays. As the name implies, a bioassay is a test that relies on a biological indicator rather than chemically determined values. In assaying the toxicity of a substance a bioassay may yield more valuable information than a chemical analysis. This is because a bioassay assesses the potential of the complete substance to cause biological harm rather examining discrete chemical units.
In general, a bioassay is a test in which a living organism is exposed to the sample in question for a discrete period of time. Many times a sample will be tested in a series of bioassays that are conducted on different life stages of a certain organism or on organisms from many different phyla spanning several trophic levels. The question to be answered by the bioassay might be: Can the eggs of fish species X survive and hatch while exposed to water from the discharge of an industrial plant? Or will fry of fish species Z survive 96 hours in the same sample? If the eggs of fish X do not hatch or fish Z does not live for 96 hours, then it is a fair assumption that there is a substance or substances in the sample that are toxic to the fish (the test would include controls to make sure the fish can survive in water that is known to be good).
However, a bioassay does not identify the toxic substance(s). Only through chemical analysis and more thorough testing can the exact toxic substance(s) be identified.
Sea urchin eggs and larvae have been used in bioassay tests for over 20 years (Jonczyk 2001). Standard experimental procedures have been developed and published by the U.S. Environmental Protection Agency (EPA 2002) that are used by most laboratories. There are many varieties of these tests that include acute and sublethal toxicity testing. Sea urchin bioassays are commonly used to test the toxicity of industrial effluents into the ocean, to examine sediment pore water, and the effects of run-off into the ocean, to name only a few.
In this report, sea urchin fertilization bioassays were used to access the survival and development of newly fertilized sea urchin eggs in freshly prepared samples of several brands of synthetic sea salts and samples of natural seawater. A previous report (Hovanec and Coshland, 2004) detailed the analysis of fifteen trace elements in these same brands of synthetic sea salts and samples of natural seawater.
Methods and Materials
The toxicity tests were conducted according to the EPA method 1008.0 Sea Urchin, Arbacia punctulata, Fertilization Test, Section 15 in Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters To West Coast Marine and Estuarine Organisms (EPA 2002). Modifications to this method are noted below.
Sea urchins. Adult White Sea urchins (Lytichinus pictus) were purchased from Marinus Scientific (Long Beach, CA) and held in 37 L aquaria filled with Catalina Seawater and located in a constant cold room (temperature=10°C) until used in the experiments. Males were held separately from females once sex was determined. The sea urchins were constantly supplied kelp collected from the beaches at Malibu and Venice, CA and Red Ogo purchased from Sea Dwelling Creatures (Los Angeles, CA). Water conditions in the aquaria were monitored twice a week for salinity, pH, ammonia, nitrite and nitrate. Water changes using fresh Catalina Seawater were performed as needed. Each aquaria had a fluorescent light which was on a 12 hours on; 12 hours off cycle.
Collection and fertilization of gametes. Fourteen sea urchins were selected from 10ºC holding tanks and placed oral side up in a Petri dish lined with several seawater-moistened paper towels. Urchins were injected with 0.5 M KCl using a 3 ml syringe with a 27.5 gauge needle through the soft periostomal membrane of each sea urchin. Each urchin received between 0.2cc to 0.5cc depending on its size. After each injection, the needle was rinsed with hot tap water to avoid accidental injection of male gametes into female gametes and vice versa. Sea urchins were held oral side up and gently swirled to induce spawning. The urchins were then placed oral side down in a tray lined with seawater-moistened paper towels. Gametes were collected for the first 15 minutes after each urchin started releasing. If spawning did not occur within 5 or 10 minutes, a second injection of 3/10cc of 0.5 M KCl was injected. If spawning still did not occur, the urchin was not used.
(i) Collection of sperm. Semen was collected dry (directly from the surface of the sea urchin), using a 200 µl pipette with the end of the tip cut off so that the opening was at least 2 mm. The semen from each male was then placed into separate 1.5 ml micro centrifuge tubes and stored in an ice water bath.
(ii) Collection of eggs. The initial release of eggs was rinsed off with Catalina seawater and disposed of as the initial release is thought to contain urchin waste. The remaining eggs were rinsed off from each individual female into 50 ml beakers with Catalina seawater. This is referred to as the egg solution.
(iii) Checking for sperm viability. In a counting chamber, a dab of semen was combined with 1 ml of Catalina water, and then examined at 400x under a Zeiss microscope in light phase. Semen was deemed viable when at least 90% possessed a tail that moved in a spiral motion causing a wiggling movement.
(iv) Checking for egg viability. A small sample of eggs from each female was placed into a counting chamber and then examined at 100X under a light microscope. Eggs were considered normal if they were round and revealed a pronucleus. Eggs were deemed viable when at least 90% were considered normal.
(v) Verification of fertilization. To check for fertilization, a sperm solution (a.k.a. dilution) was necessary. In a 1.5 ml epi 50 µI of sperm from all the males was combined and vortexed. In a 50 ml falcon tube, 12.5 µL of pooled sperm was placed into 25 ml of seawater. This solution was then stored in an ice water bath for no more than 1.5 h. Every 1.5 h a new sperm solution was made. Fertilization was tested for each individual egg solution with the sperm solution. In a 1 ml micro centrifuge tube, 1000 µL of sperm solution and 375 µL of egg solution were combined. After 20 min fertilization was checked. Fertilization was positive when a fertilization membrane formed around the eggs. Sperm-egg pairs were accepted when at least 90% of the eggs acquired a fertilization membrane.
(vi) Pooling and dilution of eggs. Once the eggs were deemed viable, they were pooled together and kept at 10ºC. Eggs were placed into a 1 L flask filled to 600 ml with Catalina seawater. After 2 h of settling, 300 ml of seawater was siphoned off with a sterile 25 ml pipette and the volume was brought up to 500 ml with Catalina seawater. This is referred to as the egg suspension solution.
(vii) Density determination. To determine the density of the sperm in the solution 25 ml of the 50 ml of freshly made sperm solution and 1 ml of 10% acetic acid was placed into a 50 ml volumetric flask. The volume was brought up to 50ml with Catalina seawater and mixed by inversion. 10 µL of killed sperm solution was then placed on each side of the hemocytometer and allowed to settle for 15 minutes. Eighty small squares on each side of the hemocytometer were counted under 400x magnification. For the density of the sea urchin eggs, 10ml of the well mixed egg suspension solution was placed into a 1 liter graduated cylinder that was brought to 1 L volume with Catalina water. This is referred to as the egg dilution. One ml of well mixed egg dilution was placed on a counting chamber and counted at 100x magnification. The process was repeated and the mean of two counts was taken.
(viii) Making of fertilized egg solution. The recommended initial sperm to egg ratio for fertilization of eggs is 500:1. The composition of this solution was determined by using the equations set forth in the Environmental Protection Agency method (EPA 2002). The initial fertilized solution was comprised of 200 ml of the egg suspension, 520 ml of Catalina Seawater and 18.8 ml of sperm solution. After 20 minutes only 5% of the eggs were fertilized. Another 10 minutes passed with no further progress. In an attempt to achieve at least 90% fertilization, more sperm solution was added into the fertilization mixture, with negative results. The final attempt was to transfer the remaining concentrated sperm into the fertilization mixture and wait 18 minutes. This was successful as 100% of the eggs were fertilized.
Synthetic sea salt treatments. The synthetic salt salts were purchased from a variety of distributors of fish and pet supplies. The synthetic salt salts tested in the first bioassay were BioSea Marine Mix, Coralife, Crystal Seas Marine Mix Bioassay, Instant Ocean, Red Sea, Reef Crystals, Tropic Marin and Oceanic Sea Salt. A natural seawater product, Catalina Sea Water, was purchased from Pete’s Tropical Fish in Simi Valley, CA. In the second bioassay Instant Ocean, Reef Crystals, Catalina Sea Water and Oceanic Sea Salt were tested.
One liter of each of the synthetic sea salts was made by dissolving an appropriate amount of the sea salt in deionized water (DI) and adjusting the salinity to 33 ppt that matched that of Catalina Seawater. The pH was not adjusted for any solution but was measured after each solution was made up and again after 24 h, just prior to the commencement of the test. All sea salt mixtures were made the day before the experiment began and allowed to sit overnight at room temperature without aeration.
For toxicity testing, 10 ml of each treatment was added to a glass vial. There were four replicates of each treatment. Treatment vials were numbered such that researchers determining larvae development did not know the identity of the actual treatment in the vial.
Toxicity test procedure. The toxicity of the sea salts was assayed by adding 250 µl of the well mixed fertilized egg solution to each vial already containing the various sea salt treatment solutions using a pipette tip that was cut to provide an opening of at least a 0.5 mm. The vials were not mixed after introducing the eggs. All vials were maintained at room temperature near a closed laboratory window that allowed natural light into the room for 72 hours. Air temperature was continuously monitored and ranged from 18.8 to 24.6°C during the 72 hours test period. The vials were exposed to laboratory fluorescent lighting of approximately 8 h light:16 h darkness.
After 72 h sea urchin development was arrested by adding 500 µl of 1.0% glutaraldehyde to each vial. Each vial was tightly capped and incubated for 1 day at room temperature. The next day
all but about 1 ml of overlying liquid was removed from each vial. The embryos in the treatments were examined under 100x magnification by two researchers over the next three days after
the glutaraldehyde was added. The first 100 embryos seen in each vial were examined and scored as being normal, abnormal or undeterminable.
Copper toxicity testing. A bioassay on the effect of copper on the development of the sea urchin larvae was also conducted as part of this experiment. A stock solution of copper was made by adding 0.4233 g of CuCl2 (anhydrous) to 100 ml of DI water. 150 µl of this stock solution was then added to 100 ml of DI to make the working stock solution. Five treatment concentrations (3.0, 6.6, 13.9, 20.4, and 30.0 µg/L Cu) were made up by adding 100, 220, 460, 680 and 1,000 µl, respectively, of working solution to volumetric flasks and diluting each with Catalina Sea Water to a total volume of 100 ml. There were four replicates of each treatment. Fertilized sea urchin eggs were added to each vial as described for the sea salt experiment. Vials were number coded and mixed in with the vials of sea salts such that the researchers scoring the vials did not know the identity of the vials with copper.
Results and Discussion
Of the fourteen sea urchins injected, five were female, seven were male, and two were not determinable. Four of the five female urchins were used, as one urchin was unable to produce enough gametes for use in the test. Sperm from six of the seven male urchins were used, as one of the urchins did not produce enough sperm. Fertilization was deemed viable in all of the sperm egg combinations used for this experiment. Sperm counts were within 80% of each other and the mean was determined to be 19,150,000 sperm/ml. For the sea urchin eggs, the mean of two counts was 3,600 eggs/ml.
Determination of whether a sea urchin larva was normal or abnormal can be subjective. Figures 1 and 2 show normally developed urchin embryos. They are characterized by:
- having lateral arms at half the length of the body,
- guts sectioned into three parts,
- “belly” portion of the anterior lateral arms present although the anterior lateral arms are not completely developed,
- overall “arrowhead-like” appearance, and
- crisp sharp image.
Figures 3 and 4, on the contrary, show abnormally developed urchin embryos. They are characterized by:
- a visible fertilization membrane,
- dark blobs of cells,
- missing any section of the gut,
- larvae still at their blastula or gastrula stage,
- missing lateral arms, and
- rods growing in abnormal directions.
Figure 5 shows an embryo that falls in between the clearly normal or clearly abnormal. These urchins are past their blastula and gastrula phase, they possess all the characteristics of being normal but their body parts are not fully developed. Perhaps these urchins are normal, but just slow in their development. Because these were difficult to categorize, the “in between” embryos may have either been counted as normal or abnormal depending on the researcher.
The pH values for the treatments on the days the salts were mixed and 24 h later just before the start of the test are presented in Table 1. Sea urchin larvae have been shown to experience poor fertilization, development and survival at high pH values (>9.0). None of the treatments had pH values that would be considered detrimental to sea urchins.
Table 1. pH values of the synthetic sea salts used in Bioassay Test 1 one hour after they were mixed and 24 h later just before the fertilized sea urchin eggs were added
| Sea Salt Brand | pH on day sea salt made | pH 24 h later just before start of bioassay |
|---|---|---|
| BioSea Marine Mix | 8.57 | 8.60 |
| Coralife | 8.13 | 8.18 |
| Crystal Sea Marine Mix | 8.51 | 8.60 |
| Instant Ocean | 8.15 | 8.18 |
| Red Sea | 8.68 | 8.69 |
| Reef Crystals | 8.15 | 8.19 |
| Tropic Marin | 8.21 | 8.26 |
| Catalina Sea Water* | ND** | 7.96 |
** not determined
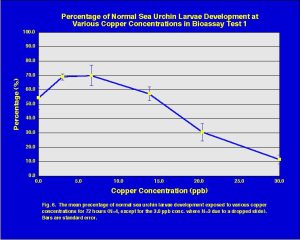
The results (mean of four replicates with standard error bars) of the copper toxicity test are presented in Fig. 6. The toxicity trend shows the classic dose response curve indicating decreased percent normal sea urchin development with increasing copper concentrations. The importance of this chart is that it shows that the fertilized sea urchin eggs were viable and do respond to increased concentrations of specific toxins.
In Fig. 7 the data from Fig. 6 is combined with the results from two other studies on copper toxicity to sea urchins. One of the primary reason for the detailed protocols, such as those published by the EPA, is so that one can compare their results with others who have previously done the same test. While, of course, there will be some variation in the results between tests, researchers and facilities, these types of comparisons are useful because they allow one to know if their results are reasonable as the results should be similar to what other researchers have found. As seen in Fig. 7, the copper toxicity curve from this study agrees well with the copper toxicity curves from the two other studies. These results tell us that our techniques are scientifically acceptable as we compare well with other laboratories that have run the same types of bioassays.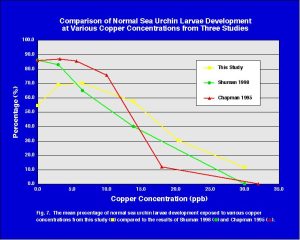
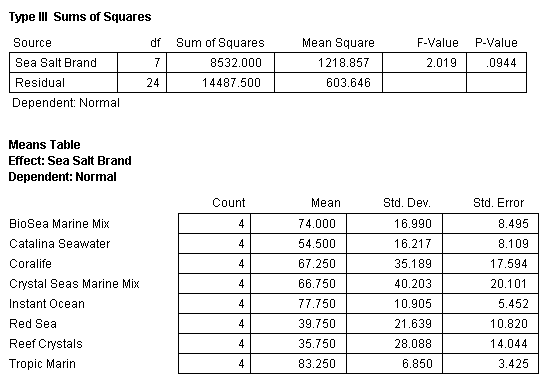
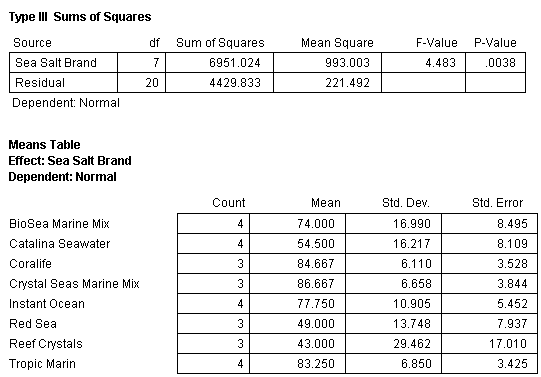
The mean percentage normal sea urchin larvae development after 72 hours for seven synthetic sea salts and one natural seawater product with standard error bars (N=4) tested in bioassay 1 is shown in Fig. 8. A statistical analysis of the data using analysis of variance (ANOVA) was conducted and determined that the means of the eight treatments were equal (Table 2). This indicates that there were no differences between any of the treatments in terms of mean percentage of normal sea urchin egg development.
Table 2. ANOVA of all the treatments and replicates in bioassay test 1 showing no significant differences between any of the treatments. Note that in this and subsequent ANOVA tables the P-Value (P stands for probability) for a significant difference is 0.05 or less. Generally, a level of 5% (0.05) or less is set by scientists as signifying that the test results show a significant trend or difference. For instance, in bioassay test 1 the null hypothesis is “The mean percentage of normal sea urchin development will be equal in all the treatments”. To reject the null hypothesis, the P-value would need to be 0.05 or less. The value determined in this test is 0.0944 and therefore the null hypothesis is accepted – all the treatments are the same.
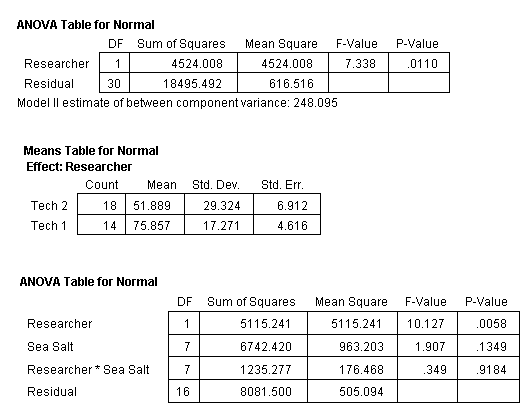
However, note that the data in Table 2 shows a large difference in the standard error values of the eight treatments (this can also be seen in Fig. 8). Further investigation into the data revealed a possibility of there being a significant difference in the scoring between the researchers. The samples were read by one of two researchers in a blind format. This means that they did not know what sample they were scoring. Each had the same guide on larvae development from which to rate the sea urchins. But was one researcher “tougher” than the other?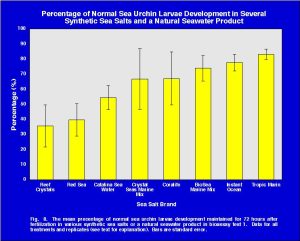
Even though the researchers randomly picked the samples, samples from each treatment were read by each of them (meaning no one individual read all four replicates from one treatment). This allowed a statistical analysis of the results to determine if there was a significant difference between researchers. Table 3 provides the results of an ANOVA analysis comparing the researchers to each other. The analysis shows that there was a significant difference between the two researchers with the percent normal egg development values of Researcher 2 being consistently lower than the values determined by Researcher 1. It seems that Researcher 2 was a tougher scorer. This means that any bias, if indeed there were a bias, would be towards a lower percentage of normal sea urchin development.
Table 3. ANOVA of all treatments and replicates in bioassay test 1 with Researcher (Tech 1 or Tech 2) who scored each sample taken into consideration. Results show a significant difference between the scoring of the two researchers, the P-Value is much less than 0.05.
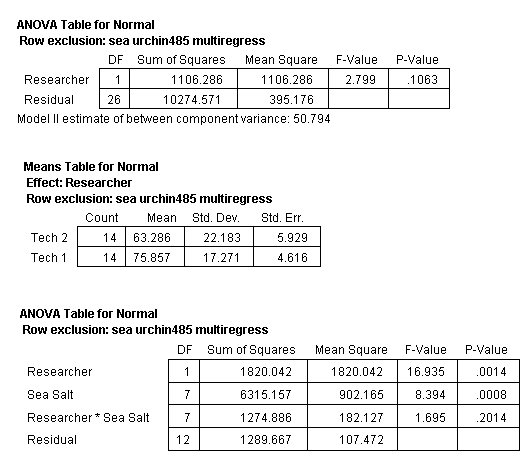
Next, the set of values for each treatment were tested for outliers (Anderson, 1987). This analysis determined that four values (one value from each treatment of Coralife, Crystal Sea Marine Mix, Red Sea, and Reef Crystals) were outliers. Each of these values was the lowest value within their treatment set. These four values were excluded from the data set and the ANOVA recalculated. The ANOVA determined that the significant difference between the two research technicians disappeared (Table 4).
Table 4. ANOVA of all treatments in bioassay test 1 but with one outlier of each of the treatments of Coralife, Crystal Sea Marine Mix, Red Sea and Reef Crystals removed with Researcher considered.
Table 5. ANOVA of all treatments in bioassay test 1 but with one outlier from Coralife, Crystal Sea Marine Mix, Red Sea and Reef Crystals removed from the analysis.
The ANOVA of the SSS and NSW was also recalculated with the above four data points removed (Fig. 9). This analysis determined that the mean normal value of the sea urchin larvae development was not equal in all the treatments (Table 5). However, this analysis does not disclose which treatments are different from the others.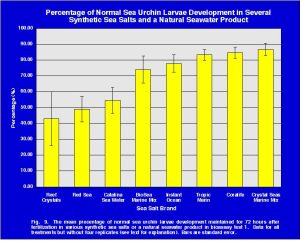
To determine which samples were different, an analysis called the Tukey Honestly Significant Difference (Tukey HSD) with the Spjotvoll-Stoline modification due to unequal sample size among treatments was conducted. This test compares the means of each treatment in a certain order starting with the largest mean against the smallest mean. Once those comparisons are finished the second largest mean is compared with the next smallest mean and so on (Zar 1974). The results can then be presented as means that are considered equal.
The result of this analysis splits the treatments into two groups (Fig. 10). The first group, which are statistically equal, consists of Reef Crystals, Red Sea, Catalina Seawater, BioSea Marine Mix, Instant Ocean and Tropic Marin. When Reef Crystals (the lowest scoring treatment) is not considered in the analysis, as per the standard procedure of this test, the means of all the remaining treatments (Red Sea, Catalina Seawater, BioSea Marine Mix, Instant Ocean, Tropic Marin, Coralife and Crystals Sea Marine Mix) are considered equal (Fig. 10).
Fig. 10. Graphical summation of the comparisons of individual group differences using Tukey HSD with unequal N (Spjotvoll-Stoline test) for the sea salt treatments(N=4)*
| Reef Crystals* | Red Sea* | Catalina Seawater | BioSea Marine Mix | Instant Ocean | Tropic Marin | Coralife* | Crystal Sea Marine Mix* |
|---|---|---|---|---|---|---|---|
| —– | —– | —– | —– | —– | —– | ||
| —– | —– | —– | —– | —– | —– | —– |
* There were only three replicates used in the analysis for these treatments.
The results from the above experiment demonstrate that SSS are not “poisonous” to sea urchin larvae. Furthermore, no one SSS can be considered better, or worse, than the others. While one might be tempted to consider the sea salts who returned lower mean values of normal sea urchin development to be “worse” than others, this view is not supported by the statistical analysis.
Furthermore, a significant difference could only be determined if certain values were excluded from the analysis. While this seems to be justified on the basis that it did eliminate the difference between the scoring of the two researchers, it also increases the chances of making a Type I error. In statistics, a Type I error is when one incorrectly rejects the null hypothesis. In this experiment, the null hypothesis is that the mean percentage normal sea urchin development of all the treatments is equal and the null hypothesis is true if the four replicates are not removed from the analysis. Overall what this means is that there is very little, if any, difference in the performance of the various SSS and NSW in the first bioassay test.
A second bioassay was conducted to investigate Oceanic Sea Salt a new sea salt that came on the market after the first bioassay had been completed. This test was run in exactly the same fashion as the first bioassay except only one researcher (the second one who was “tougher”) read all the vials.Three synthetic sea salts (Instant Ocean, Reef Crystals and Oceanic Sea Salt) and one natural sea salt product (Catalina Sea Water) were included in this test for a total of four treatments.A copper toxicity test was also performed as part of this bioassay (Cu concentrations of 6.6, 13.9 and 30.0 µg/L were used).
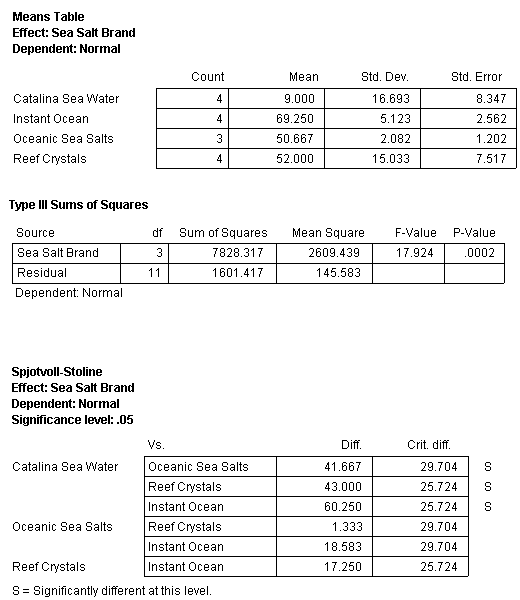
The initial pH values of the four treatments were normal (data not shown). The means, standard deviation and standard error for the second bioassay are presented in Table 6. One vial (replicate) of the Oceanic Sea Salt treatment was dropped during counting so there were only 3 replicates for this treatment. The other treatments had four replicates each. Analysis of the results (ANOVA) showed that the percent normal development in the three synthetic sea salts (SSS) was significantly different (better) compared to the Catalina Sea Water but there were no differences between the three SSS.
Table 6. Mean, standard error, ANOVA and posthoc analysis (Tukey HSD with Spjotvoll-Stoline modification) of results in bioassay test 2. N equals 4 (except for Oceanic Sea Salts N=3)
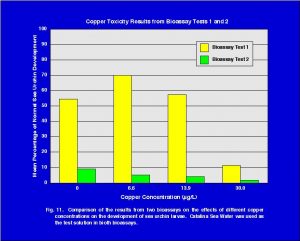
The mean percent normal development of sea urchin larvae in Catalina Sea Water was 9.0. This value was the lowest of all the treatments in the two bioassays. Further evidence that this batch of Catalina Sea Water was toxic to sea urchin larvae comes from the results of the copper toxicity testing. Catalina Sea Water was used as the sea water solution in the copper toxicity testing of both bioassays. A comparison of the copper results from the two bioassays is shown in Fig. 11. The second copper bioassay with Catalina Sea Water showed very poor development for all concentrations of copper. This is in contrast to the results of the first bioassay (Fig. 6) and other bioassays (Fig. 7), which have demonstrated that at low concentrations of copper sea urchin larvae development normally. While the results of the second bioassay clearly show that the second batch of Catalina Sea Water was toxic to sea urchin eggs/larvae, it does not tell us why. Trace element or other chemical analyses were not conducted on this sample so one cannot speculate as to the definitive reason this sample was toxic.
The statistical results for the three SSS show that they were not significantly different in terms of percentage normal sea urchin development (Table 6). For comparison purposes the mean values for the treatments of bioassay 2 and the values for the same treatments in bioassay 1 are presented in Fig. 12.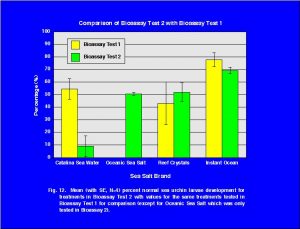
The results of the two bioassays demonstrate that the eight brands of synthetic sea salts do not significantly retard the development of sea urchin larvae that have spent their first 72 hours after fertilization in these mixtures. The results for the natural seawater product Catalina Sea Water were mixed. In one bioassay, there was no significant effect on the sea urchin larvae development. However, in the second bioassay there was almost no normal development of the sea urchin larvae in Catalina Sea Water.
Of course, the more important questions, which can only be discussed and not definitively answered, are: How relevant are sea urchin bioassay results to marine aquaria? How can one correlate or extrapolate the results of these tests to the survival of organisms in marine aquaria? As previously mentioned, many times bioassays are conducted on many different life stages of an organism and on many different trophic levels in an environment. Research has shown that fish eggs can be much more susceptible to certain toxins than adults of the same species, while the toxicity to fry falls in between the two extremes. However, generalizations are difficult because much relies on the actual species and environmental conditions. For example, copper is known to be much more toxic in soft freshwater (below 50 mg/L CaCO3) versus hard water (above 150 mg/L CaCO3).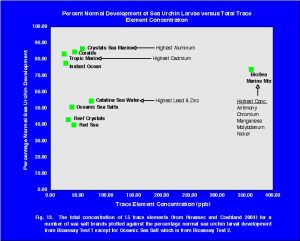
None of the synthetic sea salts examined in this test severely retarded the development of newly fertilized, in contrast to the natural seawater product Catalina Sea Water, and thus there is no evidence to suggest that the synthetic sea salts in these tests would be detrimental to organisms in marine aquaria including reef tanks.
Of course, this report only includes the results of two bioassays and only a portion from each bag of SSS was utilized in the bioassays. Two potential criticisms might be that:
- an investigator should mix up the entire bag of sea salt and take a sample from that mixture to run the test and
- the bioassays should be conducted multiple times over a longer time period of time to cover different production runs and the possible variation that comes with any manufacturing process.
To the first point, modern manufacturing processes are used to completely blend the SSS on the market these days. Further, many
times only a portion of a bag of SSS is used at one time for doing small water changes etc. Thus the procedures used in the bioassays do reflect how SSS are used in many situations.
A survey of the scientific literature can be used to address the second point regarding conducting multiple bioassays. A review of a number of seawater toxicity tests published in the journal of SETAC (Society of Environmental Toxicology and Chemistry) reveals that between the years of 1996 into early 2004, 43 studies were published that used a synthetic sea salt (Fig. 14). Of these 43 studies, 35 of them used Instant Ocean with the next most common SSS being Crystal Sea Marine Mix that was used in 4 studies. Fig. 14 also presents a breakdown of the tests by organism group in which Instant Ocean was used. If a SSS were toxic it would quickly be discovered from all the aquatic testing that occurs in laboratories from around the world. This wide range of experiments and organisms used in the tests conducted in multiple laboratories is ample evidence that SSS, especially Instant Ocean, are not toxic to aquatic organisms and the hobbyist can rest assured that they are maintaining their marine aquaria with a high quality product.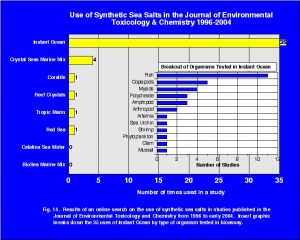
Furthermore, in one of the 43 studies mentioned above, Jonczyk et al (2001) compared natural seawater to a synthetic sea salt in a large series of sea urchin fertilization bioassays that spanned 4 years. These investigators used the white sea urchin, Lytichinus pictus, in a series of bioassays split into two phases to determine if there were any differences between the two marine waters when used to adjust salinity in bioassays or when the tests were conducted in 100% of these waters. They compared Instant Ocean to Atlantic Ocean water and Pacific Ocean water and found that when comparing hypersaline brine to dry salts “it is our view that the dry salts (Instant Ocean) method should be used for adjusting salinity in these tests.” When comparing the sea urchin fertilization toxicity tests when either natural seawater or synthetic seawater is used to dilute a number of industrial effluents they conclude that synthetic seawater (Instant Ocean) prepared from dry salts can be satisfactorily used for control/dilution water is these tests.” Finally, when examining the results for the bioassays on copper conducted in natural seawater and synthetic sea salts (Instant Ocean) they found “that fertilization rates are significantly higher when using artificial water for dilution rather than natural seawater.”
In conclusion, the results of this study and many other studies demonstrate that there is no evidence of toxicity of freshly mixed synthetic sea salts to sea urchin larvae and no evidence that using synthetic sea salts in a marine aquarium would be detrimental to the health of the organisms in the aquarium.
Literature Cited
- Anderson, R.L. 1987. Practical Statistics for Analytical Chemists. Van Nostrand Reinhold New York. 316p.
- Chapman, G.A., D.L. Denton, and J.M. Lazorchak.1995. Short-term methods for estimating the chronic toxicity of effluents and receiving waters to West Coast marine and estuarine organisms. EPA/600/R-95-136. Cincinnati, OH: U.S. Environmental Protection Agency. 661p.
- EPA. 2002. Short-term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Marine and Estuarine Organisms. 3rd Edition EPA-821-R-02-014.
- Hovanec, T. A. and J. L. Coshland. 2004. A chemical analysis of select trace elements in synthetic sea salts and natural seawater. Advanced Aquarist Online www.advancedaquarist.com Sept. 2004.
- Jonczyk, E., G. Gilron and B. Zajdlik. 2001. Sea Urchin Fertilization Assay: An evaluation of assumptions related to sample salinity adjustment and use of natural and synthetic marine water for testing. Environ. Toxicology and Chemistry 20(4): 804-809.
- Pilson, M.E.Q. 1998. An Introduction to the Chemistry of the Sea. Prentice-Hall, Inc. Upper Saddle River, N.J. 431p.
- Shuman, C.S. 1998. Evaluation of the US EPA Purple Sea Urchin, Strongylocentrotus purpuratus, Fertilization and Larval Development Test Methods. Master of Arts Thesis, University of California, Santa Barbara. 43p.
- Zar, J.H. 1974. Biostatistical Analysis. Prentice-Hall, Inc. Englewood Cliffs, N.J. 620p



0 Comments