Last month we presented the results from our experiments comparing deep sandbed and plenum-based aquaria under controlled conditions in the laboratory as Part 1 of this series. Although those results are very useful in understanding the real effects of the different aquarium designs, few people are really interested in keeping a tank without any live animals. This month, we will continue our experimental comparison of sandbed and plenum-based systems under more realistic conditions. This second experiment uses live rock, fish and invertebrates along with the full complement of natural sandbed infauna found in similarly-sized sediments on Hawaiian reefs to evaluate the relative nutrient processing capacity of sandbeds of various depths and grain sizes with and without a plenum beneath them. In
this article we will explain our live animal experiments, and present the results of this experiment to scientifically examine the relative contribution of: (1) a plenum void-space (sandbed with or without plenum); (2) the sediment depth of the bed (2.5 versus 9.0 cm); and (3) the mean particle size of sediments in the bed (2.0 versus 0.2 mm mean particle diameter) to their nutrient processing capacity and performance as a lone filtration method for recirculating aquaria.
We presented the background for this study in Part 1 of this series, and will not repeat it here. If you have not already done so, please read Part 1 for the relevant introduction to this work.
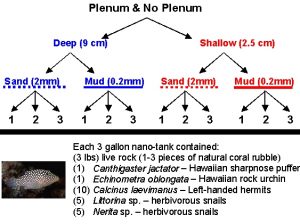
Figure 1a: Schematic of aquarium design experiment to compare directly the effects of the presence or absence of a plenum, the depth of the sediment bed, and the mean particle size of sediments in recirculating aquarium systems.
Experimental Methods & Materials:
a) Aquarium set up and nutrient dosing
As described in the previous article, we set up a factorial design experiment with three replicate nano-tanks (27 cm long x 17 cm wide x 30 cm high) for each factor: with or without plenum, deep or shallow, and coarse or fine sediments for a total of 24 experimental aquaria (Fig. 1a,b). Unlike the previous experiment, the live animal trials were run under natural conditions which were quite variable through time. Aquaria were maintained outdoors in a shade enclosure to protect the aquaria from inputs of rain and direct sunlight, but both temperature and light fluctuated at natural rates. During the experiment, the maximum air temperature recorded was 33ºC (~91ºF) and the minimum air temperature recorded was 19ºC (~66ºF); aquarium temperatures varied less than these extremes, and ranged from 22 and 30ºC (~72 to 86ºF).
Sediments from the previous experiment were removed from each tank and combined together along with an equivalent volume of natural sediments of equivalent size collected from the lagoon at Coconut Island (Hawaii Institute of Marine Biology, Kaneohe, HI). These sediments, along with the natural infauna community, were mixed thoroughly by hand and then redistributed among the aquaria for each treatment. As with the previous experiment, the deep sandbed treatments contained 9.0 L (~2.4 gallons) of wet sediment to provide a constant depth of roughly 9.0 cm (~3.6″). Shallow sandbed treatments contained 2.5 L (~0.7 gallons) of wet sediment to provide a constant depth of roughly 2.5 cm (~1″). Florida crushed coral gravel (#0, mostly oblong, averaging ~2x4mm, with a mean particle diameter ~2.0 mm) from the first experiment was mixed with similarly sized particles collected from around the reefs in Kaneohe Bay, Hawaii. Southdown Tropical Play Sand (mean particle diameter ~0.2 mm) from the first experiment was mixed with similarly sized sediments collected from the lagoon at Coconut Island in Kaneohe Bay, Hawaii. Sediments of each type were thoroughly mixed before being redistributed among the treatment aquaria (Figure 1b).
Treatments were assigned to the same aquaria as in the first trial, and were set up in the same way as outlined in Part 1. After sediments were distributed among aquaria, the tanks were allowed to settle for 1 week without any nutrient additions prior to the introduction of live animals. Water was circulated using a CAP-180 powerhead as described previously for the dosing experiments. Water parameters were tested (see below) for each aquarium at the end of this week to determine the starting conditions for each trial aquarium.
After the 1 week stabilization period, we added 1 kg of “live rock” (consisting of 1-3 pieces of natural coral rubble collected from the nearby reef), a single Hawaiian sharpnose puffer (Canthigaster jactator), a small rock urchin (Echinometra oblongata), ten left-handed hermits (Calcinus laevimanus), and 10 snails (5 Littorina sp. and 5 Nerita sp.) to each aquarium. Although this sounds like only a light stocking level, it is important to keep in mind that the nano-tanks used for this experiment were only 3 gallons. Also, the deep sediment trials were half filled with sand leaving only half the aquarium volume for water and animals. If we were to scale this stocking level up to a 50 gallon tank, we’d have 50 lbs of live rock, 90g of fish (roughly equivalent to 8 or 9 adult yellow tangs), 16 golf-ball sized urchins, 220 hermit crabs, 220 snails and all the natural infauna associated with a natural coral reef environment. Clearly when you think of the stocking level on that scale, each tank contained a decent bioload relative to a well-stocked reef tank.
We prepared homogenized squid (Loligo sp.) pellets for food as described in Pawlik et al. (1995). Fish were fed ad libitum each week day (unfed on weekends) until they did not ingest the final pellet offered to them. The final uneaten pellet was left in the aquarium to provide food for scavengers in the tank. The number of pellets fed to each tank therefore differed from day-to-day and tank-to-tank. The final number of pellets fed to each tank was different at the end of the experiment. However, over the course of the entire experiment, there were no significant differences in the number of squid pellets fed per treatment. Any deaths of animals in the aquaria were recorded at each testing period, and replacement animals were added as necessary to maintain a constant bioload of the same number of live animals in each treatment throughout the experimental period.
The experiment ran for 118 days after the addition of live animals without any water changes. Again, the salinity of each aquarium was adjusted to ~53 mS every other day as outlined in Part 1.
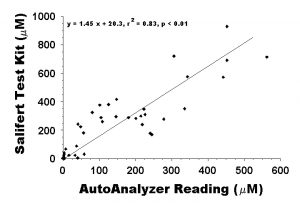
Figure 2: plot of the readings obtained from the Salifert aquarium test kits used to record daily nutrient levels presented, and the readings provided from the Water Chemistry Analysis Lab at the University of Hawaii at Manoa using a Technicon AutoAnalyzer. For direct comparison, the readings obtained from the Salifert test kits (mg/L) are converted into the same concentration units as those obtained from the Technicon AutoAnalyzer (µM).
b) Aquarium water testing
All tanks were initially filled from a large holding tank of well-mixed natural seawater taken from the seawater system at the Hawaii Institute of Marine Biology. A single 50ml sample of this water was collected and frozen at -80°C until water analyses were completed at the end of the experiment. Likewise, a single 50ml sample from each aquarium was collected at the end of the experiment and also frozen at -80ºC. At the completion of both portions of the experiment (this experiment and the laboratory dosing experiment outlined in Part 1 of this series), all water samples were transported frozen to the University of Hawaii at Manoa and water nutrient concentrations were determined using colorimetric methods on a Technicon AutoAnalyzer as outlined in Laws et al. (1999).
Each experimental aquarium was also tested twice or three times per week for salinity, pH, ammonia, nitrite, nitrate, oxygen, phosphate, calcium, alkalinity, and organics using standard aquarium testing equipment from an online aquarium supplier. Salinity was determined using an electronic PinPoint salinity meter (calibrated to 53.0 mS using IAPSO seawater) and pH was measured with the electronic PinPoint pH probe (after 2-point calibration to 7.0 and 10.0). All other water parameters were measured using standard Salifert aquarium test kits as outlined in Part 1.
c) Statistical analyses
If you are not familiar with statistics at all, you will probably want to skip this section. It won’t stop you from being able to read the rest of the article, but we present the details here for those readers who want to know how the analyses were done.
We performed all statistical tests using JMPin ver. 4.0.2 Academic Version (SAS Institute Inc.). We performed a bivariate linear fit of aquarium test kit readings (response variable) to the AutoAnalyzer readings (factor), and tested the significance of the Lack of Fit test as implemented in JMPin. All other statistical analyses were carried out using an Analysis of Variance as implemented in JMPin. We first confirmed conformity to assumptions of normality using Shapiro-Wilks, and homogeneity of variance using Bartletts test (alpha = 0.01) before performing the ANOVA analysis. The full ANOVA model used as the presence or absence of a plenum, the mean particle size of sediments, the depth of the bed and interactions among them as fixed effects; the salinity, pH, ammonia, nitrite, nitrate, oxygen, phosphate, alkalinity, and calcium were measured as response variables. Significant differences among treatment pairs (plenum vs. none; fine vs. coarse particles; deep vs. shallow sediments) were determined for each response variable through effect tests as implemented in JMPin. Data were plotted using PSI Plot ver, 7.01 (Poly Software International, Inc.).

Figure 3: Time series plot of the mean ammonia concentration (mg / L) in experimental aquaria. Note that the ammonia concentrations throughout the entire experiment are lower than those observed in the dosing experiments reported in Part 1.
Our Experimental Results
a) Live animal aquarium experiments
Time-series of ammonia, nitrite and nitrate concentrations in aquaria showed little difference among treatments (Figs. 3-5). As with the dosing experiments presented in Part 1, the time-series of pH, salinity, ammonia, nitrite and nitrate concentrations in aquaria showed no significant differences among treatments (data not shown). Analyses of variance for each water parameter revealed no significant differences among the final salinity, ammonia, nitrite, oxygen, or organic concentrations, nor were there any significant interactions among experimental treatments for any of these water parameters (data not shown). There were significant differences among treatments for the remaining water parameters and the variances are uniformly larger among treatments including live animals than in the dosing experiments (i.e., there is much more variation from tank-to-tank when live animals are included in the aquarium).

Figure 4: Time series plot of the mean nitrite concentration (mg / L) in experimental aquaria. Again, readers should note that the nitrite concentrations throughout the experiment are lower than those observed in the dosing experiment presented in Part 1.

Figure 5: Time series plot of the mean nitrate concentration (mg / L) in experimental aquaria. It is noteworthy that peak nitrate levels in the live animal experiments are equal to those observed in the dosing experiments presented in Part 1, but this may be a limitation of the Salifert test kits which have a maximum reading of 100 mg / L. Overall, the concentrations of nitrate recorded throughout the experiment are significantly lower than those reported for the dosing experiments (similar to the results for ammonia and nitrite).

Figure 6: Comparison of final nutrient concentrations in experimental aquaria with and without plenums. Bars represent the mean concentration among tanks with (blue cross-hatch) and without (red cross-hatch) a plenum beneath the sediments. Error bars are standard errors among replicates, and parameters that show a significant difference between a DSB and plenum design are flagged with an asterisk. Salinity is measured in mS, alkalinity in meq, and organics are presented as a relative colorimetric measure. Nitrate, calcium, oxygen, ammonia, phosphate and nitrite are all presented in mg / L. Treatments which differ significantly between the live animal and laboratory dosing experiments are highlighted with a bar above them.
By the end of the experiment, pH was significantly higher in aquaria with fine (8.22 ± 0.02 SE) than coarse (8.10 ± 0.02 SE) sediments (df = 1, F = 7.68, p = 0.01). For nitrate, the overall analysis of variance was not significant (df = 7, F = 1.25, p = 0.34). However, there was a significant particle size by depth interaction effect (df = 1, F = 6.48, p = 0.02), in which deep, coarse (27.41 mg / L ± 6.89 SE) and shallow, fine (20.42 mg / L ± 6.89 SE) sediments have the highest average final nitrate concentration, while shallow, coarse (12.08 mg / L ± 6.89 SE) and deep, fine (0.67 mg / L ± 6.89 SE) sediments consistently had the lowest final nitrate concentrations. Phosphate ended up significantly higher in aquaria with coarse (0.35 ppm ± 0.02 SE) than fine (0.02 ppm ± 0.02 SE) sediments (df = 1, F = 119.69, p< 0.01). Phosphate was also significantly higher among deep (0.22 mg / L ± 0.02 SE) than among shallow (0.15 mg / L ± 0.02 SE) sediment treatments (df = 1, F = 5.70, p = 0.03), although this comparison becomes non-significant after Bonferroni correction. Alkalinity was significantly higher in tanks with fine (1.97 meq / L ± 0.06 SE) than with coarse (1.69 meq / L ± 0.06 SE) sediments (df = 1, F = 12.03, p < 0.01). Finally, calcium concentrations were significantly higher in tanks with fine (340.42 mg / L ± 2.89 SE) than with coarse (327.92 mg / L ± 2.89 SE) sediments (df = 1, F = 9.35, p < 0.01).

Figure 7: Comparison of final nutrient concentrations in experimental aquaria with deep (9.0cm) and shallow (2.5cm) sediments. Bars represent the mean concentration among tanks with deep (blue) and shallow (red) sediments. Error bars are standard errors among replicates, and none of the parameters show a significant difference between deep and shallow sediments. Salinity is measured in mS, alkalinity in meq, and organics are presented as a relative colorimetric measure. Nitrate, calcium, oxygen, ammonia, phosphate and nitrite are all presented in mg / L. Treatments which differ significantly between the live animal and laboratory dosing experiments are highlighted with a bar above them.
No other source variables or interaction terms were significant, however all but one of the water parameters tested (see below) showed similar trends to the dosing experiments presented in Part 1. Overall most comparisons that were significant in dosing experiments presented in Part 1 were also close to significant (0.1 < p > 0.05) despite the higher variability in live animal trials presented here. This similarity shows that the addition of live animals to the experiment had little overall effect on the results. Given that similarity, and the increased variability with the inclusion of live animals, an increased sample size for the live animal experiments would almost certainly have shown identical trends among the two experiments. In fact, the only parameter that showed an opposite effects between the dosing and live animal experiments was alkalinity in the presence or absence of a plenum (Fig. 6).
b) Aquarium water testing
Comparisons of the nutrient concentration in initial and final water samples determined by AutoAnalyzer to the results obtained from the Salifert aquarium test kits were sufficiently well-correlated (r2 = 0.75, F = 10.19, P < 0.01) to use the aquarium test kit values as a relative measure of aquarium nutrients throughout the experiment. This correlation (also presented in Part 1) is a direct comparison of the readings from the aquarium test kits (in mg/L) with the concentration readings from the AutoAnalyzer (in µM). However, that is not actually a fair comparison. If we convert the results from the aquarium test kits to µM concentrations (for a direct comparison of the accuracy of the test, rather than the relative magnitude as reported by the correlation above), the correlation is actually better (r2 = 0.83, F = 5.41, P < 0.01, Fig. 2). Although there is a decent correlation between the values obtained with an AutoAnalyzer and the Salifert aquarium test kit, the overall Salifert test kit readings (143.5 ± 25.4) were significantly higher on average than were the AutoAnalyzer (84.7 ± 15.9) readings (df = 1, F = 356.0, p < 0.01). Although this is encouraging because it leans toward the conservative (high) side for the test results, it is important to note that the difference between readings could be more than double or less than half the true value (Fig. 2 above).
So, as long as you use the test kits as a ballpark figure for the concentration of nutrients in the aquarium, this kit performs quite reliably (we did not test any other kits, and cannot comment on their accuracy). However, there is considerable variability among readings given by the Salifert kit, and if you are interested in a highly accurate reading of nutrients in the aquarium, I suggest you read Dana Riddle’s article on Water Testing Devices for Advanced and Professional Aquarists in the Advanced Aquarist archives.

Figure 8: Comparison of final nutrient concentrations in experimental aquaria with coarse (2.0mm mean diameter) and fine (0.2mm mean diameter) particles. Bars represent the mean concentration among tanks with coarse (purple) and fine (green) sediments. Error bars are standard errors among replicates, and parameters that show a significant difference between particle sizes are flagged with an asterisk. Salinity is measured in mS, alkalinity in meq, and organics are presented as a relative colorimetric measure. Nitrate, calcium, oxygen, ammonia, phosphate and nitrite are all presented in mg / L. Treatments which differ significantly between the live animal and laboratory dosing experiments are highlighted with a bar above them.
c) Death rates among live animal treatments
We also kept track of all animal deaths in the live animal experiments. Each animal in the experiment was treated as equivalent, and the total number of individuals that had to be replaced throughout the experiment was compared. We compared the mean death rate for animals: 1) among experimental treatments (Fig. 9), and 2) by similarity to popular aquarium designs (Fig. 10).

Figure 9: Comparison of mean death rate of all animals (fish, snails, urchins & hermits combined) among experimental treatments. Treatments that do not differ significantly from one another are labeled with the same letter above each bar (only shallow sediment trials are significantly higher than deep sediment trials).
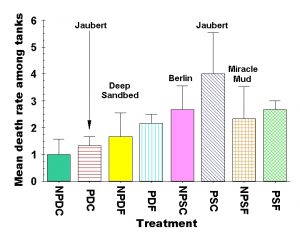
Figure 10: Comparison of mean death rate of all animals (fish, snails, urchin & hermits combined) among experimental treatments most closely resembling the setup of popular aquarium design methods. The treatments are abbreviations of the plenum, sandbed depth and sediment grain size in that order. NP denotes “no plenum” while P denotes a “plenum” is present. D stands for “deep” beds (9.0 cm) whereas S stands for “shallow” (3.0 cm) beds, and C denotes “coarse” sediments (2.0 mm) while F denotes “fine” (0.2 mm) sediment beds. It is critical to note that we did not actually test any of the aquarium designs named in this figure – we are simply indicating which design most closely matches one of the experimental treatments we used in our experiment for readers to compare. Typical plenum designs would be halfway between PDC and PSC in depth, and “Jaubert” is therefore labeled twice on the figure, because the expected mortality rate would be halfway between these extremes.
For the among treatment comparison, the overall analysis of variance was not significant (df = 7, F = 0.88, p > 0.5). However, there were nearly twice as many animal deaths overall in shallow as in deep sediment tanks (Fig. 9). On average 2.91 ± 0.46 animals had to be replaced in the shallow sediment treatments, whereas only 1.47 ± 0.46 animals had to be replaced in the deep sediment trials (df = 1, F = 5.23, p < 0.05). No other treatment or interaction term significantly affected thedeath rate in our experiment.
If we examine the overall death rates among all depth, particle size and aquarium design combinations, we find there are considerable differences in the mean death rates among some of the treatments. If we examine the aquarium design in our experiment that is most similar to some of the common aquarium designs, we can see some interesting differences (Fig. 10). Although we never intended to set out to test all possible aquarium setup designs, the factorial approach we have taken gives us experimental tanks that are close in design to many of the popular aquarium set ups. This allows us to compare the relative performance of shallow coarse sediments (such as used in the Berlin aquarium design) to shallow fine sediments (such as used in the Miracle Mud aquarium design). Clearly these specific designs have other components (such as a skimmer or Miracle Mud) that would be required to directly compare these designs to those of a deep sandbed or plenum design. However, it also seems reasonable to us that a skimmer could be added to any of these tanks and therefore show a different effect, so the relative comparison of the sediments themselves (as used in the most popular aquarium setups currently) is worth some attention. We have labeled the sediment treatment that is closest to each popular aquarium design in Figure 10 for comparison. The Jaubert plenum design uses an intermediate depth sediment bed, so we have labeled both the deep and shallow plenum design with “Jaubert.” Given our results, we would expect the true Jaubert plenum design to fall roughly halfway between these two extremes.
Overall, deep coarse sediments without a plenum had the lowest death rate in the experiment (1.0 ± 0.58 animals replaced), while shallow coarse sediments above a plenum had the highest overall death rate (4.0 ± 1.53) in the experiment. Shallow coarse sediments without a plenum (2.67 ± 0.88) and shallow fine sediments with (2.67 ± 0.33) or without (2.33 ± 1.20) a plenum were all on the higher end of the death rates recording in the experiment as well (Fig. 10). These higher mortality sediment combinations are closest in design to those of the Berlin (NPSC) and Miracle Mud (NPSF) systems (Figure 10).
Discussion & Conclusions
As we explained in Part 1, public aquaria and hobbyists at home have long used recirculating systems based on some form of sediment filtration to aid in the processing of nitrogenous wastes produced by tank inhabitants (reviewed by Delbeek, Sprung, 1994a; b; Carlson, 1999; Toonen, 2000a; b; Borneman, Lowrie, 2001; Delbeek, Sprung, In press). The design of these sediment filtration units for recirculating systems to culture coral reef organisms fall largely into only a few major types: Berlin, Miracle Mud, plenum and sandbed-based systems. However, these systems can also be viewed as a simple continuum from virtually no sediment with complete reliance on live rock and protein-skimming in Berlin systems, to extreme amounts of sediment and no skimmer with some deep sandbed systems. Despite the diversity of opinions on the value of these different designs, the relative utility of each of these types, and the most effective means to design them are still a subject of considerable controversy (reviewed by Toonen, 2000a; b).
There have been some studies to compare the relative performance of a given design (e.g., Auger, 1999; Hovanec, 2003), however to date these studies have all been unreplicated and only show results based on comparisons from a single aquarium of each design. In Part 1, we showed how even in a replicated experiment set up in a laboratory without any live animals and with ammonia dosed in to simulate an identical bioload among tanks, there is far too much variability to draw any conclusions based on a single tank. We reiterate the point made by Terry Siegel in his editorial last month: we need experimental evidence to draw any intelligent conclusions about the relative advantages of any particular aquarium design or additive.
The varied opinions and continuous debate of this subject were what led us to begin this experiment a couple of years ago, and here we finally present the experimental data that compare directly a variety of recirculating nano-reef aquarium designs. We performed a controlled and replicated factorial design experiment to determine the relative effect of the presence or absence of a plenum, the depth of the sediments and the size of the particles in the sandbed on the concentration of nutrients in the aquarium. Put simply, our experiment shows that the presence of a plenum has no measurable benefits over simply depositing the same sediments directly on the bottom of the aquarium (at least for nano-tanks over the time scales that we tested).
With a single exception, the results of the live animal experiments were similar to those of the animal-free dosing experiments (Figs. 7-9). Only alkalinity showed the opposite pattern of significance in the presence or absence of a plenum among the dosing and live animal experiments (Fig. 7). Although final concentrations of nitrate and calcium did not vary among plenum, sediment depth or particle size treatments within either the dosing or the live animal experiments, both differed significantly between the two experiments. Nitrate concentrations of experimental aquaria in the live animal experiments (15.15 ± 17.51) were significantly lower than those of the dosing experiments (62.76 ± 14.47) (df = 1, F = 150.33, p < 0.01). Likewise, final calcium concentrations of experimental aquaria with live animals (334.17 ± 11.81) were significantly lower than those in the aquarium dosing experiments (446.67 ± 37.15) (df = 1, F = 199.95, p < 0.01).
We cannot exclude the possibility that the presence of live animals in the aquarium may alter the buffering capacity or the rate of denitrification. However, the most likely explanation for reduced final calcium concentrations is uptake by organisms in the trial aquaria which could not happen in the dosing experiments presented in Part 1. The same could be said for the nitrate concentration, but there are at least three additional potential explanations for the differences between the live animal and dosing trials in the final nitrate concentration. First, the presence of the coral rubble (‘live rock’) in the live animal trials could well have increased the biological filtration capacity, and could account for the reduced final nitrate concentrations. Second, the waste introduced to the aquarium by the live animals is likely much lower than 0.5mg NH4+ / L / day. Based on a rough calculation of size-specific nitrogenous waste production from Qian and colleagues (2001), we estimate that the rate of ammonium production in the live animal trials was probably between 0.05 and 0.08 NH4+ / L / day. Finally, the live animal trials were conducted outside beneath a shade enclosure, and the presence of algae in these treatments could easily account for significant nitrate uptake relative to the aquarium dosing treatments. Further experimentation would be required toaddress the specific cause of the reduced nitrate concentrations in the live animal trials.
To our surprise, the majority of the nutrient processing capacity appears to be explained quite simply by microbial processes. These experiments show no evidence that the presence or absence of live animals and sediment infauna have a measurable effect on the nutrient processing capacity of sediments (Figs. 7-9) – at least in nano-tanks on the time scales covered by this experiment. However, the question of how these results scale up to larger aquaria, and the role of sediment infauna in the long-term stability of closed systems certainly remains a subject for future studies. We cannot address these questions with our data, and hope that someone will follow up on this study to specifically research the question of time and scale in these systems.
Perhaps the most perplexing result from this experiment is the significant interaction of sediment particle size and depth in the aquaria. The simple prediction based on sandbed depth would be that deeper and finer sediments should always have reduced oxygen penetration and therefore increased nitrate processing capacity (Toonen, 2000a; b; Shimek, 2001; Delbeek, Sprung, In press). Therefore, it is hard to explain why deep, coarse (27.41 mg / L ± 6.89 SE) and shallow, fine (20.42 mg / L ± 6.89 SE) sediments have the highest average final nitrate concentration, while shallow, coarse (12.08 mg / L ± 6.89 SE) and deep, fine (0.67 mg / L ± 6.89 SE) sediments consistently had the lowest final nitrate concentrations. Nitrate reduction in deep, fine sediments is easily explained by reduced oxygen penetration to
the sediments. However, the increased final nitrate concentrations in aquaria with deep, coarse and shallow, fine sediments relative to the shallow, coarse treatment is harder to understand. Additional research will be required to explain the source of denitrification in shallow, coarse sediments and account for this unexpected result. During his MACNA XVI presentation, Julian Sprung discussed his research into the physical effects of water motion on the biological filtration capacity of sediment beds in aquaria. The basic conclusion from that work (covered in more detail in Delbeek, Sprung, In press) is that the location and volume of rock as well as the surface shape of the sand or gravel (e.g., mounds, sloped, or flat) can dramatically affect the efficiency of water flow, oxygen diffusion and nutrient processing in the sandbed. The results we present here likewise argue that there are complex interactions between sandbed depth, particle size and flow that are sometimes counter-intuitive. Obviously, additional research along these lines may prove very fruitful to our ultimate understanding of biological filtration in recirculating aquaria.

Figure 11: Time series of nitrate in an experimental aquarium prior to and after the death of a fish in the aquarium. The arrow on the plot marks the point at which the fish died, and the ammonia, nitrite and nitrate level all show a significant increase immediately after that event (we present only one plot as a representative data set). Overall, bioload and animal deaths in the aquarium appear to be the best predictors of water quality in these experiments.
Overall, both the results of the dosing and live animal experiments suggest that there is no measurable difference between most of these common sediment filtration designs for maintaining suitable water parameters. There were no significant differences among depth, particle size or plenum treatments for the processing of ammonia or nitrite in recirculating aquarium systems. Deep, fine sediments had the lowest average final concentration of nitrate in these trials, but these values were not significantly less than the average final concentration of nitrate in shallow, coarse sediment treatments. Also, contrary to our expectations, the presence or absence of live animals and sandbed infauna made no significant difference to the nutrient concentrations across the time periods tested here. So what does explain the differences among aquaria in these experiments? Well, it turns out that the best predictor of aquarium nutrient levels is quite simply the bioload and any animal deaths in the tanks. Aquaria that had low (even undetectable) levels of ammonia, nitrite and nitrate would suddenly show a substantial peak in nitrogenous wastes following the death of an animal in the aquarium (Fig. 11). Our results suggest that stocking level of the aquarium, and any animal deaths, have a much greater effect on the overall water quality than the specific design of the aquarium set-up you chose to follow.
Ultimately, however, we suspect that most aquarists are less concerned about the exact concentrations of any of these water parameters, and are instead acutely concerned with whether or not animals survive in their aquaria. Our experiment showed that sandbed systems had a slightly lower mortality rate than systems based on a plenum; likewise, mortality in tanks with coarse sediments was very slightly lower than those based on fine sediment, but neither effect was significant (Fig. 9). The only significant effect was that death rate in shallow sediments was significantly higher than (almost twice) that of tanks with deep sediments (Fig. 9), and the highest death rate of all was observed in aquaria with shallow, coarse sediments over a plenum (Fig. 10). Insofar as the sediments themselves are an important component of the aquarium design, these results can be used to infer the relative efficiency of a variety of aquarium designs with different depths and sizes of sediment included within them.
In conclusion, regardless of whether we look at the laboratory dosing or the live animal experiments, our results show no evidence for any of the espoused benefits of a plenum. To the contrary, these experiments suggest that any benefits seen are a direct consequence of the sediments themselves rather than the void space beneath it. However, at least over the time scales we could test, our experiments also showed little support for the espoused benefits of a natural community of infauna in the sediments. Overall, there was only one qualitatively different trend observed between the laboratory dosing and live animal trials, and that was alkalinity rather than any of the nitrogenous waste products (Figs. 6-8). This study is the first experimental comparison of aquarium designs to compare their relative performance. We cannot address any long-term effects on aquarium maintenance or survivorship in this experiment, and we hope that others will follow up on our work to address this issue. This sort of experimental data is necessary to evaluate alternative aquarium designs or additives objectively, and anecdotal evidence or unreplicated studies should always be viewed with skepticism.
Overall Summary:
- Our experiment shows no evidence for any of the espoused benefits of a plenum (reviewed by Goemans 1999) either with or without live animals in the design. Instead our results suggest that any benefits seen are a direct consequence of the presence of the sediments themselves rather than the void space beneath it.
- Each sediment-based aquarium design appeared capable of handling nutrient inputs up to 0.5 mg / L / day of NH4+ – which is equivalent to a well-stocked reef aquarium. At this input level, final concentrations of ammonia, nitrite and nitrate did not differ significantly among aquaria 1) with or without plenums, 2) containing deep (9.0 cm) or shallow (2.5cm) sediments, or 3) containing coarse (2.0mm) or fine (0.2mm) mean particle sizes. Bioload and animal deaths in the aquarium show a much greater effect on the water quality than does the specific design for the tank.
- The greatest differences among experimental treatments were observed as decreased buffering capacity, and higher final phosphate concentration of aquaria with coarse sediments relative to those with fine sediments. However, the chemical composition of the gravel may be responsible for this effect, and we have not tested other gravel types of similar size. We recommend that aquarists test any new gravel for dissolution before adding a lot of it to their aquarium.
- We show that there can be extreme variation among identical tanks, even without any live animals included as outlined in Part 1. Given the added variability as soon as live animals are included into the mix, our results highlight the problem with drawing any conclusions based on a single aquarium – no matter how beautiful it may be. The results from any study lacking proper replication and controls should be viewed with suspicion. We argue that anecdotal evidence is simply presentation of an opinion in cases such as this, and more than 5 years of heated debate on the merits of DSB vs. plenum systems has resulted from the staunch defense of opinions without data.
- We show that even high-quality aquarium tests provide only a ballpark estimate of the actual concentration of nutrients in the aquarium. However the readings from the Salifert test kits were sufficiently correlated with the true nutrient values to be reliable for comparisons among aquaria.
- Overall death rates were roughly twice as high in aquaria with shallow sediments as in deep sediment treatments. The highest overall death rates were seen in aquaria with shallow coarse sediments over a plenum, and the lowest death rates occurred in aquaria with a sandbed composed of deep coarse sediments. The treatments that were closest to the design aquarists employ for deep sandbed, Miracle Mud and Jaubert plenum aquaria had intermediate death rates. The shallow coarse sediment design that is closest to that used in Berlin systems had one of the highest death rates, and the deep coarse sediment design for which there is currently no accepted name had the lowest overall mortality (Fig. 10). We did not test bare bottom tanks, but the data clearly suggest that the shallower the sediment, the higher the mortality rate, and you can’t get much shallower than a bare bottom tank!
- Experimental results were surprisingly similar between the aquarium dosing and live animal experiments. Contrary to our expectations, the presence of live animals and sediment infauna does not have any measurable effect on final nutrient concentrations in our experimental aquaria.
Acknowledgements
This research was funded in part by a Program Development Award to RJT from Hawaii Sea Grant. Additional funding came via donations from Reed Mariculture, Catalina Aquarium and my very understanding wife, Carol Fong. Water testing was performed by Saipologa Toala and Houston Lomae as part of a Pacific Islander Undergraduate Mentorship in Environmental Biology (UMEB) internship, and we greatly appreciate their diligence and hard work assisting with this project. We thank Ross Shaw for taking photographs of our aquarium setups. This manuscript was improved by discussion and comments from Eric Borneman, Anthony Calfo, Charles Delbeek, Tom Frakes, Richard Harker, Tim Hovanec, Larry Jackson, Julian Sprung and the many other excellent aquarists at the XVI Marine Aquarium Conference of North America.
References
- Auger, P., 1999. The quantitative comparison of two nutrient removal systems. St. Mary’s College, Baltimore, MD.
- Borneman, E.H., Lowrie, J., 2001. Advances in captive husbandry and propagation: An easily utilized reef replenishment means from the private sector? Bulletin of Marine Science 69, 897-913.
- Carlson, B.A., 1999. Organism responses to rapid change: What aquaria tell us about nature. American Zoologist 39, 44-55.
- Delbeek, J.C., Sprung, J., 1994a. The Reef Aquarium, Vol. 1. Ricordea Publishing, Coconut Grove, FL, 544 pp.
- Delbeek, J.C., Sprung, J., 1994b. The Reef Aquarium, Vol. 2. Ricordea Publishing, Coconut Grove, FL, 546 pp.
- Delbeek, J.C., Sprung, J., In press. The Reef Aquarium, Vol. 3. Ricordea Publishing, Coconut Grove, FL.
- Hovanec, T.A., 2003. A comparison of coral reef filtration systems: preliminary results. SeaScope 20, 1-3.
- Laws, E.A., Ziemannb, D., Schulman, D., 1999. Coastal water quality in Hawaii: the importance of buffer zones and dilution. Marine Environmental Research 48, 1-21.
- Pawlik, J.R., Chanas, B., Toonen, R.J., Fenical, W., 1995. Defenses of Caribbean sponges against predatory reef fish. I. Chemical deterrency. Marine Ecology Progress Series 127, 183-194.
- Qian, P.-Y., Wu, M.C.S., Ni, I.H., 2001. Comparison of nutrients release among some maricultured animals. Aquaculture 200, 305-316.
- Shimek, R., 2001. Sand Bed Secrets: The common-sense way to biological filtration. Marc Weiss Companies, Inc., 36 pp.
- Toonen, R., 2000a. Are Plenums Obsolete? Another viewpoint, Part 1. Freshwater and Marine Aquarium (FAMA) 23, 44-66.
- Toonen, R., 2000b. Are Plenums Obsolete? Another viewpoint, Part 2. Freshwater and Marine Aquarium (FAMA) 23, 44-70.




0 Comments