This month, our discussion of Super Corals continues with a focus on an attractive stony coral Montipora undata. To my knowledge, this coral has no common name.
With proper conditions, this Montipora species can grow quickly, and its green fluorescence along with reddish-purple tips and white polyps makes it appealing.
Note that observations presented in this report are of only one specimen containing an unknown zooxanthella clade. However, there is still a lot of information available. We’ll begin our discussion with the ‘types’ of zooxanthellae known to infect M. undata specimens.
Montipora undata contains at least one fluorescent pigment (green) and a non-fluorescent chromoprotein (purple). Polyps appear white under full spectrum lighting.
- Family: Acroporidae
- Genus: Montipora
- Latin Name: Montipora undata (see Basis of Identification below)
- Geographical Range: Indian and Pacific Oceans.
- Known Symbiont Types: Symbiodinium species, Clade C1 and Clade C·.
Symbiont Types
Clade C1
Chen et al., (2005) found this clade in Taiwan M. undata specimens at depths of 3-5m. Clade C1 is considered a ‘generalist’ zooxanthellae in that it infects a large number of coral genera hosts in the Pacific as well as the Atlantic, and is distributed over a wide variety of depths. Thus, corals containing this clade could be considered highly adaptive as they can tolerate high light (but are probably best suited for lower light intensities). Evidence seems to indicate that these zooxanthellae photo-saturate at 200 – 400 µmol·m²·sec or less (or 15,000-20,000 lux; see below). Clade C1 (along with C3, C21, C3d, C1c and C45) is believed to be an ancestral type from which other clades evolved (LaJeunesse, 2004) and has been found in these corals: Acropora cervicornis (Baker et al., 1997), Acropora divaricata, A. humilis, A. hyacinthus, A. longicyathus (from the GBR; van Oppen et al., 2001), Acropora palifera (from Taiwan, Chen et al., 2005), Acropora sarmentosa , A. tenuis (GBR; Van Oppen et al., 2001), Astreopora (GBR, LaJeunesse et al., 2003), Astreopora myriophthalma (Taiwan; Chen et al., 2005), Caribbean anemones Bartholomea and Condylactis spp. (LaJeunesse et al., 2003), Great Barrier Reef ‘corallimorpharia’ and Coscinaraea, Coscinaraea wellsi and Cycloseris vaughani from Hawaii (LaJeunesse et al., 2004), Cyphastrea (LaJeunesse et. al., 2003), Cyphastrea chalcidicum (van Oppen, 2005), Atlantic and Pacific Discosoma spp. (LaJeunesse, 2005), Echinophyllia orpheensis, Echinophyllia lamellosa (Chen, 2005), Caribbean Eunicea (LaJeunesse et. al., 2003), Euphyllia ancora, Euphyllia glabrescens (Chen, 2005), Favia (LaJeunesse et. al., 2003), Favia favus and Favites abdita from Taiwan (Chen, 2005), Fungia (LaJeunesse et. al., 2003), Fungia crassa (van Oppen, 2005), Galaxea, Goniastrea (LaJeunesse et. al., 2003), Goniastrea rectiformis (Chen, 2005), Goniopora (LaJeunesse et. al., 2003), Goniopora columba, Goniopora lobata (Chen, 2005), Herpolitha, Hydnophora (LaJeunesse et. al., 2003), Hydnophora excessa (Chen, 2005), Icilogorgia, Lebruna, Leptastrea (LaJeunesse et. al., 2003), Leptoria phrygia (Chen, 2005), Leptoseris incrustans (LaJeunesse, 2004), Linuche, Lobophytum, Merulina (LaJeunesse et. al., 2003), Merulina ampliata (Chen, 2005), Merulina scrabicula (van Oppen, 2005), Millepora sp. ((LaJeunesse et. al., 2003), Montipora aequituberculata (Chen, 2005), Montipora cactus from Indonesia (van Oppen, 2004), Montipora cactus, Montipora curta from Taiwan (Chen, 2005), Montipora confusa (van Oppen, 2004), Montipora digitata, Montipora effluorescens, Montipora hispida, Montipora sp., Montipora spongodes, Montipora undata from Taiwan (Chen, 2005), Mycedium (LaJeunesse et. al., 2003), Mycedium elephantotus (Chen, 2005), Pachyseris, Palauastrea, Caribbean Palythoa, Hawaiian Palythoa (LaJeunesse et. al., 2003), Pavona desucata, Pavona frondifera, Pavona varians, Pavona venosa (Chen, 2005), the ‘bubble’ coral Plerogyra (LaJeunesse et. al., 2003), Plesiastrea verispora (Chen, 2005), gorgonians Plexaura and Plumigorgia (LaJeunesse et. al., 2003), Taiwanese Pocillopora damicornis (Chen, 2005), Polyphyllia, Porites sp. (LaJeunesse et. al., 2003), shallow-water Porites cylindrica, Porites lutea, Porites solida (from Taiwan; Chen, 2006), GBR Psammocora (LaJeunesse et. al., 2003), Pseudosiderastrea tayamai (Chen, 2005), Rhodactis, Rumphella, Sarcophyton, Pacific Scolymia, Siderastrea, Sinularia (LaJeunesse et. al., 2003), Stylocoeniella guentheri (Chen, 2005), Stylophora sp. ((LaJeunesse et. al., 2003), Stylophora pistillata (Chen, 2005), a giant clam (Tridacna sp.), Turbinaria sp. (LaJeunesse et. al., 2003), Turbinaria mesenteria (Chen, 2005), and Pacific and Caribbean Zoanthus spp. (LaJeunesse et. al., 2003).
Clade C·
Van Oppen, 2004, reports M. undata specimens from Indonesia contain Clade ‘C·’. Clade C· is believed to have co-evolved with Montipora species, but sometimes found in Porites attenuata and Porites cylindrica. Other Montipora species containing Clade C· include Montipora aequituberculata, M. altasepta, M. angulata, M. cactus, M. capitata, M. crassituberculata, M. danae, M. delicatula, M. digitata, M. gaimardi, M. hispida, M. hoffmeisteri, M. mollis, M. peltiformis, M. spongodes, M. stellata, M. turtlensis, and M. verrucosa (van Oppen et al., 2004). This clade is presently known to be distributed from Indonesia southward to the Great Barrier Reef. One has to wonder if this clade has high-fidelity to Montipora spp. and is one of those listed in LaJeunesse’s more-or-less concurrent paper (namely Clades C17, C26a, C27, C30, C31, C31a, C31b, C32, C58 and C73). Van Oppen’s IDs are based on ITS1 sequences (while LaJeunesse’s – and many others’- are based on ITS2 DNA fingerprinting).
These works are important to hobbyists if we subscribe to the hypothesis that corals containing the same clade(s) zooxanthellae will have the same adaptive capacities to light intensity. As technology evolves, and more samples are studied, our knowledge will undoubtedly change about coral/symbiont relations. For the moment, this is the best information we have.
M. undata– How Much Light?
Second only to ‘How much is it?’ is usually ‘How much light does it need?’ And, the answer is ‘Not much, relatively speaking.’ Figure 1 shows the rate of photosynthesis of a single M. undata specimen under various light intensities (See ‘Methods and Materials’ for a description of equipment used to measure photosynthesis).
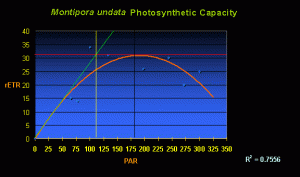
Figure 1. The rate of photosynthesis (relative Electron Transport Rate, or rETR, shown by the orange line) of a Montipora undata. In this case, maximum photosynthesis occurs at a PAR value of ~200 µmol·m²·sec. Increasing light intensity above this point is counter-productive as photoinhibition occurs.
Perhaps some explanation is needed in order to understand the chart. The curved orange line demonstrates the rate of photosynthesis (rETR, for Electron Transport Rate between Photosystem II and Photosystem I) relative to light intensity (the yellow numbers at the bottom of the chart and labeled as ‘PAR’ – for Photosynthetically Active Radiation). A quick inspection says maximum photosynthesis occurs at PAR values of about 180-200 µmol·m²·sec (or ~9,000 – 10,000 lux). The chart also has other information – that of ‘on-set of photosaturation.’ The method described by Kirk (1983) is used to determine on-set of saturation. If the initial rate of photosynthesis (characterized by the left hand side of the orange line in the chart of Figure 1) was to continue in a linear fashion (the green line), its intersection with the maximum rate of photosynthesis (the red line) is the onset of photosaturation (the vertical yellow line intersect with the PAR values at the bottom – in this case, onset of saturation is in the neighborhood of 110 µmol·m²·sec (or ~5,500 lux) with full-blown photosaturation at 180-200 µmol·m²·sec (or ~ 9,000 – 10,000 lux).
Note: The specimen in the introductory photograph is maintained under one of PFO’s very early LED prototypes.
Pigments
Some Montipora undata specimens contain at least two pigments generated by the coral animal. These include a fluorescent protein with an emission peak at ~490nm (blue-green; See Figure 2) and an unidentified non-fluorescent chromoprotein (that appears reddish-purple under full spectrum lighting. It will appear bluish under high kelvin light sources).
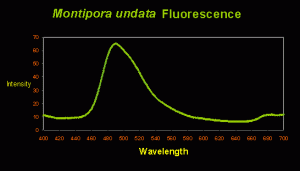
Figure 2. The blue-green fluorescent pigment has an emission peak at ~490nm.
Interestingly, Fluorescent Pigment (FP-490) has been found in only Acroporidae corals, including Acropora and Montipora species. The corals species containing FP-490 are Acropora digitifera (peak excitation at 425nm – violet light; Dove et al., 2001); Acropora millepora (excitation at 405nm – violet light; Cox and Salih, 2005); Acropora aspera (fluorescent shoulders at 501 and 514nm; excitation at 480nm – blue light; Papina et al., 2002); Acropora nobilis (excitation at 462nm – blue light; Karan et al., 2006); Montipora monasteriata (broad excitation at 420-450nm; Dove et al., 2001) and Montipora undata (this report).
In all cases, excitation wavelengths are in the violet/blue portion of the spectrum. This suggests FP-490 is best viewed under high kelvin lamps (note that UV energy also excites this fluorescent pigment and causes it to ‘glow’, but is not necessary). However, the purple-red non-fluorescent chromoprotein will appear dark blue. Supplemental warm light will make the purple apparent and, subjectively, more appealing.
I have not been able to identify the non-fluorescent protein’s maximum reflectance or absorbance. This pigment is generated by the coral animal (and not zooxanthellae) and is usually seen in high growth areas. It is uncertain if this particular pigment eventually ‘kindles’ into the fluorescent green pigment (which would explain why the pigment disappears as the growth areas phase from new to old. Much work remains to be done before we understand these pigments’ natures and purposes, if any).
Water Motion
This coral species does not seem especially demanding in its water flow requirements – flows measured in an aquarium found water velocity to be ‘average’ for a ‘real’ reef – 0.5 ft/sec (See ‘Methods and Materials’ – below – for water velocity testing protocols).
Although one half foot per second does not seem like excessive velocity (it equates to roughly 1/3 mile per hour), it does take some effort to generate this velocity in aquaria. Fortunately, the ‘propeller pumps’ now on the market make generating this sort of water flow quite easy.
Alkalinity and Calcium
Carbonate alkalinity is important for two reasons – it buffers against downward shifts of pH and also provides carbonates necessary for building the corallum or skeleton. Use any of the commercially available buffers to maintain an alkalinity of 175 mg/l (as CaCO3) or ~3.5 meq/l. Calcium concentrations of at least 400 mg/l are recommended although values of up to 450 mg/l have been observed and seem acceptable.
Temperature
Zooxanthellae clades (a clade is something like a sub-species) found in M. undata are not known to be particularly heat-resistant.To avoid potential overheating of coral specimens, water temperature should not exceed 80º F when using metal halide lamps or other lamps that operate at very high temperatures (mercury vapor lamps, for instance. See Riddle, 2006 for details). This is due to absorption of heat by the coral skeleton, where it can become warmer than ambient water temperature.
Comments
This coral will grow very quickly when conditions are correct. Specimens can double in size in just a few months.
Fragmentation of this coral is easy. It can be accomplished with a Dremel tool with ‘cut-off’ blades or with a ‘wire cutter’ pliers (preferably stainless steel).
Mahalo to Steve Ruddy with Coral Reef Ecosystems (www.coralreefecosystem.com) for his invaluable assistance in preparation of this article.
Basis of Identification
Veron’s Coral ID (2002) software was used for identification with 30 data points entered.
The ocean realm entered was Indo-Pacific. The exact collection point is unknown, so two likely locations were entered:
If the coral was collected from the Central Indo-Pacific (including the Philippines, Malaysia, Indonesia and the Solomon Islands), choices are M. undata, M. danae and M. confusa (with the software’s ‘Best Bet’ Option choosing M. undata).
If the coral was collected from the oceanic west Pacific (including the Palau, Vanuatu, Fiji, and the Marshall Islands), the software’s ‘Best Bet’ Option chose M. undata).
These are the other data points used for identification (based on microscopic examination of the skeleton and inspection of enlarged photographs of living animals):
- Colonial coral
- Attached to substrate (as opposed to free-living)
- Growth form is encrusting or solid plates (but can include columnar growths)
- Branching is absent (in this case)
- Calice width <1mm
- Corallite centers distinct
- Corallites separate
- Neither axial nor central corallites
- Corallites are widely spaced
- Corallite protrusion immersed
- Tentacles expanded during the day
- Tentacle Length <10mm
- Tissue partially masks skeleton
- Daytime tissue projection <1mm
- Columella absent
- Costae absent
- Unequal septal length
- Radial septa
- 2 cycles septa
- Septa not exsert
- No septal fusion
- Septal height: not exsert
- Septa not petalloid
- Septal margins not smooth
- Paliform structures absent
- Extra-thecal skeleton present
- Extra- thecal surface perforated
- Extra-thecal elaborations present
- Linear elaborations > calice diameter
Methods and Materials
Water velocities were measured with a Marsh-McBirney Flo-Mate 2000 electronic digital water velocity meter. This meter operates on Faraday’s Law and reports velocity with a resolution of 0.01 foot per second.
Rates of photosynthesis were determined with a Walz ‘Teaching PAM’ Chlorophyll Fluorometer (Effeltrich, Germany) equipped with a fiber optic cord. A fluorometer is basically a ‘photosynthesis meter’ and exploits measurements of chlorophyll fluorescence in order to determine rates of photosynthesis (in terms of ‘electron transport rate’ or ETR between Photosystem II and Photosystem I within zooxanthellae). Corals were maintained in total darkness for at least one hour before initial measurements were made in order to allow Photosystem reaction centers to ‘open’. The external actinic light source was a 400-watt Iwasaki 6,500K metal halide lamp that was shielded for ultraviolet radiation by a clear acrylic material. Intensity was adjusted upwards by moving the light source closer to the coral (which was contained in a 3-gallon plastic container). Light intensity (Photosynthetically Active Radiation, or PAR) was measured with an Apogee QMSS quantum meter and a submersible, cosine-corrected sensor. Water motion was provided by a magnetic stirrer and 3″ stir bar.
After the dark-adaptation period, minimum chlorophyll fluorescence (Fo) was determined with a weak pulse of light generated by the instrument’s internal actinic lamp. Maximum Fluorescence (Fm) was estimated by increasing the intensity of the internal actinic lamp, resulting in a photosynthetically-saturating pulse of light. Once minimum and maximum fluorescence values are established, the instrument can determine the relative Electron Transport Rate (rETR) under different lighting conditions.
The metal halide lamp was then turned on and allowed to ‘warm up’ until PAR values stabilized (15-20 minutes). Chlorophyll fluorescence values were determined and the light intensity increased. The coral’s zooxanthellae were allowed to adjust to the new light intensity for 15 minutes and another chlorophyll fluorescence measurement was made. This procedure was repeated multiple times, with three measurements taken at each light intensity. These results were averaged.
The PAM meter calculated Photosynthetic Yield, which was simply multiplied by the appropriate PAR measurement in order to estimate the rETR. This is a valid method for observing photosynthetic trends. Technically, it is not the preferred method of determining absolute ETR, but it is fine for our purposes.
Fluorescence was determined with an Ocean Optics USB-2000-FL fiber optic spectrometer (Dunedin, Florida) using an 18-watt black light (maximum emission at 365nm) as the excitation source. Light was collected with a cosine-corrected CC-3 lens and a 600 micron fiber optic cable.
The photograph was taken with a Canon Rebel XTi digital camera (10.1 megapixels) equipped with two stacked teleconverters and a 60mm macro lens.
References and Suggested Reading
- Baker, A., R. Rowan and N. Knowlton, 1997. Symbiosis ecology of two Caribbean Acroporid corals. Proc. 8th Int. Coral Reef Symp., Panama. 2:1295-1300.
- Chen, C., Y-W Yang, N. Wei, W-S Tsai and L-S Fang, 2005. Symbiont diversity in scleractinian corals from tropical reefs and sub-tropical non-reef communities in Taiwan. Coral Reefs, 24(1): 11-22.
- Cox, G. and A. Salih, 2005. Fluorescent lifetime imaging of symbionts and fluorescent proteins in reef corals. In: Multiphoton Microscopy in the Biomedical Sciences V, edited by A. Periasami and Peter So. Proc. SPIE, 5700:162-170.
- Dove, S., O. Hoegh-Guldberg and S. Ranganathan, 2001. Major color patterns of reef-building corals are due to a family of GFP-like proteins. Coral Reefs 19: 197-204.
- Karan, M., B. Filippa, J. Mason, S. Dove, O. Houegh-Guldberg and M. Prescott, 2006. Cell visual characterisitic-modifying sequences. US Patent US2006/0107351 A1.
- Kirk, J.T.O., 1983. Light and Photosynthesis in Aquatic Ecosystems. Cambridge University Press, Cambridge. 401 pp.
- LaJeunesse, T. and R. Trench, 2000. Biogeography of two species of Symbiodinium (Freudenthal) inhabiting the inter-tidal anemone Anthopleura elegantissima (Brandt). Biol. Bull. 199: 126-134.
- —————–, 2000b. Investigating the biodiversity, ecology and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region in search of a species level marker. J. Phycol., 37: 866-890.
- —————–, 2002. Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar. Biol., 141: 387-400.
- ——————, W. Loh, R. vanWoesik, O. Hoegh-Guldberg, G. Schmidt and W. Fitt, 2003. Low symbionts diversity in southern Great Barrier Reef corals, relative to those in the Caribbean. Limnol. Oceanogr., 48(5):2046-2054.
- —————–, D. Thornhill, E. Cox, F. Stanton, W. Fitt and G. Schmidt, 2004. High diversity and host specificity observed among symbiotic dinoflagellates in reef coral communities from Hawaii. Coral Reefs, 23:596-603.
- ———————-, R. Bhagooli, M. Hidaka, L. de Vantier, T. Done, G. Schmidt, W. Fitt and O. Hoegh-Guldberg, 2004b. Closely related Symbiodinium species differ in relative dominance in coral reef host communities across environmental latitudinal and Biogeographical gradients. Mar. Ecol. Prog. Ser., 284: 147-161.
- ——————, 2005. “Species” radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene-Pliocene transition. Mol. Biol. Evol. 22(3): 570-581.
- ———————, G. Lambert, R. Andersen, M. Coffroth, and D. Galbraith, 2005. Symbiodinium (Phyrhophyta) genome sizes (DNA content) are smallest among dinoflagellates. J. Phycol., 41: 880-886.
- ——————, S. Lee, S. Bush and J. Bruno, 2005b. Persistence of non-Caribbean algal symbionts in Indo-Pacific mushroom corals released to Jamaica 35 years ago. Coral Reefs, 24(1): 157-160.
- ———————, H. Reyes-Bonilla and M. Warner, 2007. Spring ‘bleaching’ among Pocillopora in the Sea of Cortez, Eastern Pacific. Coral Reefs, in press.
- Papina, M., Y. Sakihama, C. Bena, R. van Woesik and H. Yamasaki, 2002. Separation of highly fluorescent proteins by SDS-PAGE in Acroporidae corals. In press, Comp. Biochem. Physiol.
- Riddle, D., 2006. Temperature and the reef aquarium. Advanced Aquarist Online. http://www.advancedaquarist.com/2006/2/aafeature2/
- Van Oppen, M., 2004. Mode of zooxanthella transmission does not affect zooxanthella diversity in Acroporid corals. Mar. Biol., 144: 1-7.
- ——————, F. Palstra, A. Piquet and D. Miller, 2001. Patterns of coral-dinoflagellate associations in Acropora: Significance of local availability and physiology of Symbiodinium strains and host-symbiont selectivity. Proc. R. Soc. Lond B., 268: 1759-1767.



0 Comments