This work is rather lengthy hence an explanation on how to use it is in order. The database (containing almost 2200 entries and available here) lists coral hosts in alphabetical order. Also listed is the zooxanthella ‘type’ or clade, along with collection location, depth, and journal reference.

Photomicrograph of zooxanthellae within coral tissues. Polyps (at upper left and lower right) contain elevated numbers of Symbiodinium. This coral, a Pocillopora damicornis, was grown on a microscope slide allowing for light to the transmitted through the thin skeleton and tissues. Photo by the author.
For example, we are interested in the zooxanthella clade found in the stony coral Pocillopora ligulata. Find the coral in the database, and we find that a P. ligulata collected by Todd LaJeunesse in 2004 at a depth of 20 meters in Hawaii contains zooxanthella clade C1g. Obviously there may be more information available (more than I could squeeze into the Excel spreadsheet) so the text portion of this article lists several hundred clades in alphabetical order. Finding C1g in the text, we see that C1g is thought to have descended from clade C1 and is possibly endemic to Hawaii.
Our understanding of how corals, and more specifically their algal symbionts, react to environmental stressors continues to increase in dramatic fashion. Analyses of genetic coding of zooxanthellae (Symbiodinium species) using sophisticated scientific techniques allow insights of the degree of flexibility these dinoflagellates possess and how they exploit these adaptive capabilities. We now know there are ‘generalist’ zooxanthellae that can thrive in wide ranges of light fields and, to a lesser extent, temperatures. Other types (or clades) of Symbiodinium do best in relatively narrow ranges and are known as ‘specialists’. This fact goes a long way in explaining why, for instance, one species of Acropora will thrive in a given aquarium while another species in close proximity (and in practically the same environmental conditions) will not do well. In addition, some clades are more resistant to loss of zooxanthellae due to a number of environmental parameters.
I began building a database on zooxanthellae clades and their hosts in 2005 and 2006 and these are available here:
- Lighting by Number: “Types” of Zooxanthellae and What They Tell Us: http://www.advancedaquarist.com/2006/1/aafeature1/
- An Update on Zooxanthellae (Symbiodinium spp.) – What a Difference a Year Makes! http://www.advancedaquarist.com/2007/4/aafeature/
I decided to consolidate and update these two articles here. While these previous articles listed about 800 corals and their ‘type(s)’ of symbionts, the database presented here contains almost 2,000 entries. A correction has been made as well, those entries claiming Clade B in Acropora species attributed to van Oppen were in error, and have been removed (Robin Smith of Florida International University pointed this out, and I am indebted for his input). However, Strychar et al., (2005) report Acropora hyacinthus to harbor a Clade B Symbiodinium.
The format has changed slightly. The Excel spreadsheet did not offer enough room to adequately display clade information for publication purposes. Hence the spreadsheet is truncated with information moved to the text portion of this article. Here, clades are examined individually, with geographical range, water depth, hosts, and additional information listed.
The complete spreadsheet can be downloaded from here in PDF format.
Why should anyone be interested in a rather obscure subject such as this? After all, we know that coral animals (hosts) and zooxanthellae (symbionts) generally have a mutually beneficial relationship. We realize zooxanthellae need light and either too much, or not enough, photosynthetically active radiation will cause problems for either the dinoflagellate or the host. In the most severe cases, the coral animal will eject its zooxanthellae in a process known as bleaching. Bleaching is generally exacerbated by higher than normal water temperature and ultraviolet radiation. New information may make us re-think the symbiosis between Symbiodinium and coral animals.

Figure 1. A few of the ‘parts’ or organelles making up a single zooxanthella cell. Not shown are many other components, such as Golgi apparatus, mitochrondria, etc.
Zooxanthella Anatomy
Zooxanthellae contain specialized parts in order to maintain cell health, function, as well as photosynthesis. Figure 1 shows some of those parts, and definitions follow immediately.
Definitions
Below are some definitions to keep in the back of your mind:
- Organelle
- A specialized sub-portion of a cell.
- Chloroplast
- The organelle where photosynthesis occurs. Thylakoid bodies (arranged in thin layers called lamellae) are coated with photopigments, including chlorophyll a, chlorophyll c2, peridinin, and others. The chlorophyll content is, of course, green; however, peridinin absorbs mostly blue and some green wavelengths, thus making the zooxanthella appear golden brown.
- Lysosome
- Organelles that contain hydrolytic enzymes.
- Nucleus
- An organelle essential to cell function, such as reproduction and protein synthesis. The nucleus is surrounded by a nuclear membrane.
- Vacuole
- Small cavities within the cell containing liquid.
Advances in DNA fingerprinting have allowed researchers to identify many life forms to species and subspecies level. A handful of dedicated scientists are devoting their careers to the investigation of various types of zooxanthellae, and are generating a great deal of data. We’ve known for some time that there isn’t just a single species of zooxanthellae (Symbiodinium microadriaticum) – there are at least 1,100 described species with many subspecies (variously called clades, types, or phylotypes). There are Clades A, B, C, D, E, F, G and H (see Figure 2). Of these, Clades A, B, C, D (and to a lesser degree F and G), with perhaps hundreds of described species (see GBIF Checklist Bank) are of interest to reef aquaria hobbyists. Symbiont populations tend to follow Fisher log-normal distribution patterns characterized by ‘generalist’ zooxanthellae (common) and rare zooxanthellae (‘specialists’) hosted by specific coral species (Pochon et al., 2001). For instance, some zooxanthellae clades are tolerant of high light intensity, while others have higher thermal tolerances, and this is where it begins to get interesting to hobbyists.
Each Clade contains sub-clades, and variations of sub-clades. In the following database, zooxanthellae sub-clades are listed by an alpha-numeric designation, in some cases, a lower case letter for further refinement (i.e., C1a indicates Clade C, and the lower case letter indicates a variation of subclade 1). The following listing reports almost 190 of them (up from about 150 in previous articles).
Even with advances in DNA fingerprinting, there is a question of speciation. What genetic markers determine if a certain clade of zooxanthellae rises to the level of becoming a new species? Until integrated examinations are completed, these questions will remain unresolved (Takabayashi et al, 2004). This isn’t that much of an issue as far as hobbyists are concerned but it is causing constant revisions in the taxonomy of symbiotic dinoflagellates.
ITS1, ITS2, lsu, etc. – What Do They Mean?
Strands of DNA have ‘regions’ such as ITS1, ITS2 and many others (ITS stands for ‘internal transcribed spacer’.) Unfortunately, researchers have not standardized methods, or more specifically the part of the DNA examined (although one of the most prolific researcher – Todd LaJeunesse – has chosen the ITS2 region). Therefore, we should be careful when comparing results reported by various scientists since portions of DNA are more, or less, conserved than other parts.
However, there is general – but not universal – agreement about zooxanthellae clades. Atlantic and Caribbean corals usually contain variations of Clade B (there are exceptions of course!) with A and C making up the difference, with Clade D only rarely seen. On the other hand, Pacific corals usually contain variations of Clade C (again with exceptions to the rule). It is believed that closure of the Central American seaway by the rise of the isthmus that is now Central America created distinct zones for coral growth and zooxanthellae specialization. The survival of Atlantic corals during glaciation of the northern hemisphere (the Ice Age) depended upon adaptation resulting in co-dominant zooxanthellae clades, while southern Pacific corals enjoyed mostly tropical environs during this period and C clades dominated. See Figure 3.
Zooxanthellae Clades
As mentioned earlier, taxonomy of zooxanthellae is constantly revised, with elevation from clade level to species not particularly uncommon. In addition, there is confusion created by using cultures of zooxanthellae – Santos et al. (2001) report that zooxanthellae cultured in vitro may not be representative of the dominant in hospite zooxanthellae clade since conditions within the culture vessel may favor the growth of a sub-dominant clade. This is an important point to consider when reviewing early research works. However, this list is believed to be correct as of late 2010.
| Symbiodinium species | Clade |
|---|---|
| S. burmudense | B |
| S. californum | B |
| S. cariborum | A1.1 or A13 |
| S. corculorum | A2 |
| S. glynni | D1 |
| S. goreaui | C1 |
| S. kawaguti | F1 or F5 |
| S. linucheae | A4 |
| S. meandrinae | A2 |
| S. microadriaticum | A1 |
| S. microadriaticum subspecies condylactis | A13 |
| S. muscatinei | B4 |
| S. pilosum | A2 |
| S. pulchrorum | B1 |
| S. trenchi | D1a |
I have included zooxanthellae species under the appropriate clade heading. Trends begin to develop and suggest (but do not confirm) characteristics that are perhaps common to each particular zooxanthellae clade.
Traits of Different Clades and Why They Are Important
As mentioned, we begin to see traits common among zooxanthellae clades. Two important traits are Xanthophyll Production and production of Mycosporine-like Amino Acids (MAAs). Again, assigning characteristics found in one clade species to all zooxanthellae found within a particular clade is risky business. However, trends do seem to develop upon close examination, at least in Clade A and Clade B.
Xanthophylls are carotenoid pigments found within many species of zooxanthellae, algae and higher plants. The two xanthophylls found in some zooxanthellae are diadinoxanthin (Dn) and diatoxanthin (DT) Brown et al., 1999. Dn and DT act as a photoprotectants and shield those zooxanthellae containing them from excessively high amounts of photosynthetically active radiation in a process called Dynamic Photoinhibition. This is simply a protective measure that prevents damage to Photosystem II. In high light, Dn absorbs blue wavelengths (Jeffries, 1997) and is converted to DT, thus shunting blue light energy away from the photosynthetic apparatus. Zooxanthellae with the ability to produce xanthophylls are equipped to endure higher light intensities with a lessened chance of destruction of their light harvesting proteins. (It should be noted that other energy dissipation pathways may be available such as release of non-radiant heat by the Photosystem II Reaction Center, or perhaps spillover of energy from Photosystem II to Photosystem I.)
While xanthophylls protect zooxanthellae from visible light energy, mycosporine-like amino acids (MAAs) protect them from ultraviolet radiation. So named because these amino acids were first isolated from fungi, MAAs are produced by plants, fungi and some bacteria. The chemical pathway leading to MAA production (the shikimate pathway) is not known to occur in animals, so MAAs can be obtained from zooxanthellae known to produce them. MAAs can also be obtained through dietary means (ingestion of algae or animals containing accumulated MAAs). Interestingly, Shick et al. report that the temperate sea anemone Anthopleura elegantissima obtains certain MAAs from ocular lenses in fishes it ingests. It is possible that bacteria and/or cyanobacteria can translocate MAAs, or modify translocated or ingested MAAs. It is also possible that translocated MAAs could be modified by the host coral). In short, MAAs can be obtained from sources other than zooxanthellae. However the ability to produce and release these important compounds to the coral host likely gives the coral a competitive edge in shallow environments. It is now believes that most, if not all, zooxanthellae clades produce MAAs upon exposure to sufficient amounts of ultraviolet radiation.
| MAA | Max. Absorbance (nm) | Promoted By |
|---|---|---|
| Mycosporine-Glycine | 310 | UV-B |
| Palythine Serine Sulfate | 320 | PAR |
| Palythine Serine | 320 | UV-B |
| Palythine | 320 | UV-B |
| Mycosporine-NMA: Serine | 325 | UV-B |
| Mycosporine-NMA: Threonine | 328 | UV-B |
| Mycosporine -2 Glycine | 331 | UV-B |
| Palythinol | 332 | UV-B |
| Porphyra | 334 | UV-B |
| Shinorine | 334 | UV-B |
A recent and very interesting paper (Banaszak et al., 2006) discusses the likelihood that many (if not all) zooxanthellae clades can produce natural sunscreens to protect themselves and their hosts from ultraviolet radiation. These researchers now believe that major clade groups (A, B, C, D and E) can produce these colorless, protective substances called mycosporine-like amino acids (MAAs). This contradicts previous beliefs based on research conducted with symbionts isolated from hosts and then cultured under relatively low light intensity (~70 µmole photons·m²·sec). It now seems that higher light intensities and/or ultraviolet radiation are needed in order for the zooxanthellae to make these pigments (the coral host cannot, since shikimate pathway is known to occur only in plants).Hence, should high-intensity lamps (such as metal halide and mercury vapor) not be shielded with a UV-absorbing lens? No – the results of Banaszak’s research only reinforces the notion that we should shield our aquarium inhabitants from potentially harmful UV radiation. For instance, a coral, grown in a dimly lighted portion of an aquarium, could be exposed to relatively intense UV radiation if it is moved only a few inches into a ‘brighter’ spot. These researchers also note the production of MAAs is an energetically expensive process (they quote a figure that 19% of a cell’s total energy budget is required for production of the MAA Palythine – energy that otherwise could be used for growth and reproduction).
In addition, it is now believed that all symbiotic zooxanthellae have, to varying degrees, the ability to produce xanthophylls. Xanthophylls (diadinoxanthin and diatoxanthin) act as photoprotectants, absorbing visible light (mostly in the violet/blue portion of the spectrum) and ‘dumping’ this energy as non-radiant heat. In effect, the conversion of these two xanthophylls under conditions of high light intensities act as a ‘safety’ valve and channel light energy away from the photosynthetic apparatus in zooxanthellae.
Why Are Some Zooxanthellae Resistant to Bleaching?
This question begs an answer why do some corals perform better than others at higher light intensity and/or temperatures and seem immune from the effects of radiation? There are many reasons why a coral could be resistant to bleaching:
- Protection from UV Radiation. A review of early literature would suggest that some zooxanthellae are not capable of producing mycosporine-like amino (MAAs) and are therefore subject to potential harm of ultraviolet radiation. Since then, the scientific opinion has shifted – all zooxanthellae clades tested were able to produce MAAs in response to relatively high amounts of UV (the zooxanthellae would often not produce MMAs in response to low UV doses).However, see the comments in the discussion section below as to why we should shield corals from UV.
- Protection from Intense Light. Some zooxanthellae are able to produce and incorporate xanthophylls to protect themselves from high light intensity. Not all do, and there are alternative protective pathways such as spillover or non-radiant heat dissipation once absorbed light energy enters the reaction center of Photosystem II (See Riddle, 2004b for details of high light intensity on captive corals). However some zooxanthellae apparently possess little, if any, means of coping with high light intensity. They will either do well in darker environments or merely survive in a hostile environment.
- Thylakoid Membrane Composition. Recent research suggests even more strategies to resist bleaching. Tchernov (2004) suggest the lipid saturation of the hydrophilic thylakoid membrane within the chloroplast determines resistance to compromise. In effect, the very composition of the light-collecting apparatus predetermines resistance to photodestruction and bleaching. Further, Diaz-Almeyda et al. (2011) determined the melting points of thylakoid membrane-bound lipids in Clades A1, A2, B1, C1 and F2.
- Absorption of Heat. A newer paper by Fabricius (2006) found that darker pigmented corals can potentially gain radiant heat and become warmer than the surrounding water temperature. Obviously this could make the zooxanthellae potentially more susceptible to a bleaching event (this happens in aquaria too – see Riddle, 2006.): http://www.advancedaquarist.com/2006/2/aafeature2/
- Hydrogen Peroxide Production. Suggett et al. (2008) found Symbiodinium clade B1 (not tolerant of high temperature) produced more hydrogen peroxide than clade A1 (less sensitive to higher temperature) when exposed to a temperature of 32°C (89.6°F). H2O2 is destructive to tissues and excessive amounts play a part in the bleaching process.
Parasitic Zooxanthellae?
Kahng and Maragos (2006) reported stony corals Leptoseris hawaiiensis and L. yabei flourishing at a depth of 120 meters (393 feet). Other reports list zooxanthellate corals surviving at depths of 165 meters (540 feet). A recent paper (Wagner et al., 2010) found black corals collected at depths of 396 meters (~1,300 feet). How do these zooxanthellae obtain their nutrition? Is it from the water column, or does the relationship become one of parasitism, where the dinoflagellate robs the animal of its resources? Perhaps we’ll have an answer as more research is conducted.
It should be noted that the concept of parasitic zooxanthellae is not new – see Stat et al., 2008 for their comments. These researchers believe Clade A zooxanthellae (found in Hawaiian Acropora hyacinthus) could compete with co-occurring Clade C for resources from the coral, thus becoming parasitic to the animal. In another hypothesis, perhaps Clade A never fully achieved a mutualistic relationship with the coral.
There is evidence that some clades become seasonally parasitic. Thornhill et al., 2008, found B2 may become photosynthetically inactive during adverse conditions, and may switch from mutualism to commensalism, or parasitism.
Coral Fidelity to Its Symbiont
While the idea that corals may adapt to changing environmental conditions by ‘shuffling’ zooxanthellae is an intriguing one, it seems to be the norm for the host to retain a specific symbiont. This has been found to be true for corals used in transplantation experiments: Fungia scutaria specimens retained their Pacific zooxanthella clade decades after transfer to the Caribbean; Hawaiian Porites compressa did the same when transferred from depth to the shallows.
Scattered reports of adaptation by symbiont shuffling could perhaps be due to cryptic symbionts becoming dominant after an upset (as good as modern technology is, identification of zooxanthellae to clade level is not reliable when a sub-population levels falls below 5-10% of the total population).
However, it has been established that dominance of a certain clade within a coral can be only temporal. For instance, young GBR Acropora tenuis specimens can contain Clades C1 and D. Clade D is dominant in younger corals and when populations shift to C1, the corals grow more quickly, suggesting the algal/host association can sometimes come at some cost (Abrego et al., 2008).
Timing is (or May Be) Everything
Venn et al. (2008) found shifts in cladal populations of Symbiodinium hosted by the anemone Condylactis gigantea to adjust to seasonal variations in temperature. In near shore environments subject to thermal variations, Clade A was predominant, while those anemones in more thermally stable environments hosted mostly Clade B. In addition, Clade A was most prevalent during periods of warm water temperature (26.5°C). During the winter, when water temps dropped to 18.5° C (65.3°F), the major population shifted to mostly Clade B. These researchers also found Clade B bleached at 32°C (89.6°F), while Clade A did not.
Clade Nomenclature
Unfortunately, there is not a universally recognized protocol for identifying different zooxanthellae clades. Generally, however, a clade is identified by an alphanumeric tag – a primary capitalized alphabetical symbol (A, B, C, etc.) followed by a numerical ID, sometimes a lower case letter and, rarely, a second lower case letter (as in C3ha). Not all researchers have followed this code and have labeled newly discovered strains by a capitalized letter and a symbol unique to that clade (e.g., C+, C·). It seems certain that most works use the former method of classification, and that the latter identification symbols will eventually conform to a widely-accepted standard.
Be aware that there are several interchangeable names for ‘clade’, including ‘group’, ‘type’, ‘phylotype’, etc. (LaJeunesse, 2001).
Clade A
Clade “A” zooxanthellae are generally considered relatively hardy (resistant to a number of environmental shifts), and are found in scleractinian corals, octocorals, hydrocorals, clams, anemones and zoanthids. Most hosts of Clade “A” zooxanthellae are found in the Caribbean, with sporadic reports of occurrences in Australia’s Great Barrier Reef, Hawaii, the Red Sea and the western Pacific (Korea). Clade A is considered ancestral to all other Symbiodinium lineages.
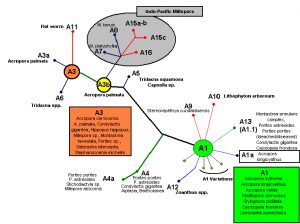
Figure 4. A phylogenic tree demonstrating relationships among zooxanthellae clades, along with host information. After LaJeunesse et al., 2009.
Interestingly, Stat et al. (2008) found that Acropora hyacinthus specimens containing Clade A were more susceptible to disease than those harboring Clade C zooxanthellae. These researchers found Clade A to fix and release only a fraction of carbon compared to Clade C. If Clade A produces only a small percentage of carbon compounds, then its very existence within the host is in danger, especially when conditions are sub-optimal. On the other hand, Stat mentions that an anemone (Condylactis gigantea) containing Clade A zooxanthellae did not suffer from any ill effects from the symbiosis.
Reported Species in Clade “A”:
Many (often early) researchers listed cladal types simply as “A”, “B”, “C”, etc.
These taxa were listed as “A”:
Acropora sp., Acropora cervicornis, Acropora formosa, Acropora hyacinthus, Acropora longicyathus, Acropora palmata, Aiptasia pallida, Anthopleura kurogane, Condylactis gigantea, Dendrophyllia, Diploria labyrinthiformis, Favia fragum, Galaxea fascicularis, Gorgonia ventalina, Hippopus hippopus, Hippopus porcellanus, Litophyton arboretum, Meandrina meandrites, Millepora sp., Montastrea annularis, Montastrea faveolata, Montastrea franksii, Montipora capitata, Nephthea sp., Plexaura homomalla, Porites astreoides (brown),Porites astreoides (green), Porites colonensis, Porites furcata, Porites nigrescens, Sarcophyton glaucum, Stephanocoenia intersepta, Stephanocoenia michellini, and Stereonephthya cundabiluensis.
Clade A1. Symbiodinium microadriaticum subspecies microadriaticum.
Geographical Range: Pandemic (Florida, Hawaii, Kenya, Great Barrier Reef)
Reported Depth Range: 0.3 – 8 meters (~1 – 26 feet)
Symbiodinium microadriaticum. Found within tissues of the jellyfish Cassiopeia and some corals, including Cassiopeia xamachana, C. andromeda and C. frondosa, Red Sea stony coral Stylophora pistillata (LaJeunesse, 2001) and Acropora cytherea, Acropora longicyathus, Acropora valida, (LaJeunesse, 2001, 2002; Visram and Douglas, 2006),and Pocillopora verrucosa. This zooxanthella species acclimates to high and low light levels. Protective xanthophylls are produced in super-saturating light intensities (this light intensity = 250 µmol·m²·sec; Iglesias-Prieto and Trench, 1997). A1 is known to produce to produce at least two mycosporine-like amino acids (mycosporine-glycine and shinorine, Banaszak et al., 2006) even in the absence of ultraviolet radiation.
This clade is considered thermally tolerant (26ºC – 78.8ºF – was the experimental temperature) by Hennige et al., 2006. Robinson and Warner (2006) also report Clade A1 is tolerant of temperature as high as 32ºC (89.6ºF), but demonstrated a reduction in photosynthetic activity as well as growth (possibly due to resources being devoted to repair of zooxanthellae photosystem(s)). Even so, Clade A1 apparently has a capacity to ‘process’ absorbed light energy (photons), thus preventing a ‘traffic jam’ of electrons between zooxanthellae Photosystems I and II, thus preventing chronic Photoinhibition (Hennige et al., 2006). In addition, Clade A1 does not produce increased amounts of hydrogen peroxide (as Clade B1 does) when exposed to elevated temperature of 32°C (89.6°F; Suggett et al., 2008).
Clade A1.1 or A13:
- Symbiodinium cariborum.
- Found in the tissues of the Caribbean anemone Condylactis gigantea.
A1a
- Reported Depth Range: 1 – 4 meters (feet)
- Reported Geographical Range: Southern Great Barrier Reef
- Host Species: Acropora longicyathus
- Reference: LaJeunesse et al., 2008.
A2
- Reported Depth Range: 10 meters and less (<33 feet)
- Reported Geographical Range: Caribbean and Pacific
- Host Species: Zoanthus sociatus, stony coral Meandrina meandrites, the photosynthetic clam Corculorum cardissa, Gorgonia ventalina, Bartholomea annulata, a Pacific hydrocoral Heliopora, and the ‘giant clam’ Tridacna gigas.
Clade A2 includes several Symbiodinium ‘species’, including:
Symbiodinium pilosum. Found in the Caribbean zoanthid Zoanthus sociatus. These are high light adapted (they respond poorly to low light levels), tolerate high temperatures swings and are able to produce and incorporate protective xanthophylls (diadinoxanthin and diatoxanthin) into chlorophyll protein complexes. Iglesias-Prieto and Trench, 1997, found this zooxanthella to be the least adaptive in respect to light intensity of 6 zooxanthellae examined (high light is tolerated while low light intensity is not).
Symbiodinium meandrinae. This zooxanthella was discovered within the tissues of the Atlantic stony coral Meandrina meandrites. It is now considered Clade A2 (LaJeunesse, 2001). Symbiodinium meandrinae. This zooxanthella was discovered within the tissues of the Atlantic stony coral Meandrina meandrites. Banaszak et al., 2000, found two zooxanthellae clades (A and C) within M. meandrites, Baker and Rowan (1997) report Clade B. This leads to confusion over the actual identity of S. meandrinae Trench (1997) clarifies the situation by listing S. meandrinae as Clade A.
Symbiodinium corculorum. Isolated from the photosynthetic Pacific clam Corculorum cardissa. Iglesias-Prieto and Trench (1997) suggest this zooxanthella species has limited photoacclimation capability and the symbiont/host perform best under high light intensity. This clam to limited to a depth of 10 meters (Gosliner et al., 1996) and is thus considered tolerant of high light. S. corculorum is now considered Clade A2 (LaJeunesse, 2001).
Besides those animals listed above, Clade A2 is also reported to be found in Gorgonia ventalina (Puerto Rico and Jamaica), the anemone Bartholomea annulata, a Pacific hydrocoral Heliopora, and the ‘giant clam’ Tridacna gigas.
A3
- Reported Depth Range: 2-15 meters (7 – 49 feet)
- Reported Geographical Range: Atlantic and Pacific
- Host Species: Known hosts include the jellyfish Cassiopeia mertensii from Hawaii (LaJeunesse, 2001; LaJeunesse et al., 2004), a Tridacna clam (species unreported, Baille et al., 2000), Tridacna crocea, T. maxima, T. derasa, T. gigas, and another ‘giant clam’ (Hippopus hippopus; LaJeunesse, 2001), Montastrea faveolata (Belize, across a depth range of 2 to 8m), a Belizean stony coral (Siderastrea intersepta, @ 8-15m; Warner et al., 2006), the anemone Condylactis gigantea, stony corals Acropora palmata, shallow-water Acropora cervicornis, Porites spp., and Stephanocoenia michelini.
- Comments: Tolerant of higher light levels (Hennige et al., 2006). A3 zooxanthellae are known to produce 1 ultraviolet-absorbing compound – the MAA mycosporine-glycine (Banaszak et al., 2006). Thornhill et al., 2008, found A3 zooxanthellae lowered their chlorophyll content when exposed to very low temperature (10.5°C – 50.9°F), and did not recover within 3 weeks of exposure (the end of the experimental period).
A3a
- Reported Depth Range: ?
- Reported Geographical Range: Philippines
- Host Species: Found in a ‘giant clam’ (Tridacna sp.)
- Reference: LaJeunesse, 2005
A3b
- Reported Depth Range: 8-15 meters (26 – 49 feet)
- Reported Geographical Range: Belize
- Host Species: The stony coral Siderastraea intersepta
- Reference: Warner et al., 2006.
A4: Symbiodinium (=Gymnodinium) linucheae.
- Reported Depth Range: 2-8 meters (7 – 26 feet)
- Reported Geographical Range: Caribbean
- Host Species: Thimble jellyfish (Linuche unguiculata). A4 is also found in the Caribbean sea whip Plexaura homomalla (LaJeunesse, 2001), Porites astreoides corals from Belize (depth of 2-8m; Warner et al., 2006) and the anemone Condylactis (LaJeunesse, 2002).
- Comments: Clade A4 is also called Symbiodinium (=Gymnodinium) linucheae.
A4a
- Reported Depth Range: 8-15 meters (26 – 49 feet)
- Reported Geographical Range: Caribbean
- Host Species: Porites astreoides, Belize 8-15m (Warner et al., 2006), the ‘fire coral’ Millepora alcicornis, anemones Condylactis gigantea and Stichodactyla helianthus (LaJeunesse, 2002).
A5
- Reported Depth Range: ?
- Reported Geographical Range: Palau
- Host Species: Tridacna squamosa and possibly the soft coral Capnella
- References: Tridacna squamosa (LaJeunesse, 2001); Pacific ‘soft coral’ Capnella (van Oppen et al., 2005).
A6
- Reported Depth Range: 1-10 meters (3 – 33 feet)
- Reported Geographical Range: Okinawa
- Host Species: Tridacna clam
- Reference: LaJeunesse et al., 2004
A7
- Reported Depth Range: ?
- Reported Geographical Range:
- Host Species: Fire coral Millepora platyphyllia
- Reference: LaJeunesse et al., 2003
A8
- Reported Depth Range: 25-30m (82-115 feet)
- Reported Geographical Range: North Great Barrier Reef
- Host Species: Millepora tenuis
- Reference: LaJeunesse et al., 2008
A9
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Acropora longicyathus
- Reference: LaJeunesse et al., 2003
A9a
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Acropora longicyathus
- Reference: LaJeunesse et al., 2003
A10
- Reported Depth Range: 7m
- Reported Geographical Range: Red Sea
- Host Species: Litophyton arboreum
- Reference: LaJeunesse et al., 2008
A11
- Reported Depth Range: ?
- Reported Geographical Range: Red Sea
- Host Species: Turbinaria sp., Stylophora pistillata and an unidentified flatworm
- Comments: A specialist zooxanthella (Barneah et al., 2007)
A12
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: ?
- Comments: LaJeunesse (2005) found this clade in an unknown coral host from a reef aquarium.
A13: Symbiodinium microadriaticum subspecies condylactis.
- Reported Depth Range: 4 – 20 meters (13 – 65 feet)
- Geographical Range: Caribbean
- Host Species: Jamaican Cassiopeia frondosa and, not surprisingly, Condylactis gigantea specimens. Also isolated from a Caribbean Porites astreoides (LaJeunesse, 2005) and Montastrea annularis.
- Comments: Thermally sensitive. Hennige et al., 2006 report this clade was stressed by higher temperature (26ºC – 78.8ºF (!) – was the experimental temperature). Robinson and Warner (2006) also report this clade is sensitive to temperature (experiment condition was 32ºC or 89.6ºF), which is exacerbated in ‘high’ light conditions.
- Clade A13 is also called Symbiodinium cariborum (LaJeunesse, 2001), as well as A1.1.
A14
- Reported Depth Range: 4 – 20 meters (13 – 65 feet)
- Reported Geographical Range: Caribbean
- Reported Hosts: Caribbean stony coral Madracis miribalis
- Reference : LaJeunesse, 2005
A15a-b
- Reported Depth Range: 3-6m (10 – 20 feet)
- Reported Geographical Range: Tanzania
- Host Species: Millepora sp.
- Reference: LaJeunesse, 2008
A15c
- Reported Depth Range: 3-6m (10-20 feet)
- Reported Geographical Range: Tanzania
- Host Species: Millepora sp.
- Reference: LaJeunesse, 2008
A16
- Reported Depth Range: 3-6m (10-20 feet)
- Reported Geographical Range: Tanzania
- Host Species: Millepora sp.
- Reference: LaJeunesse, 2008
A188*
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Reported Hosts: Caribbean corals
- *Comment: The numerical portion (188) of the clade ID is based on the length (bp) of a variable region in the chloroplast 23S rDNA gene, and not the ITS1 or ITS2 regions used by many researchers.
- Reference : Coffroth et al., 2010.
A194*
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Reported Hosts: Caribbean corals
- *Comment: The numerical portion (194) of the clade ID is based on the length (bp) of a variable region in the chloroplast 23S rDNA gene, and not the ITS1 or ITS2 regions used by many researchers.
- Reference : Coffroth et al., 2010.
- Summary: Generally, Clade A zooxanthellae seem tolerant of high light intensity, and likely produce protective xanthophylls (for protection from predominantly ‘blue’ light) and mycosporine-like amino acids (that can absorb ultraviolet energy). Its existence is sometimes correlated with shallow back reefs. The number of hosts containing Clade A zooxanthellae populations are noted to decrease with increasing depth. Some have speculated that Clade A has never achieved full mutualism with its host or, under some stressful circumstances, could become parasitic.
Clade B
As with Clade “A” zooxanthellae, those of Clade “B” are relatively resistant to bleaching episodes. Current information suggests this clade is most common in Caribbean gorgonians (sea fans, sea whips, etc.).A subclade (B1) has been found in Hawaiian Aiptasia anemones and the stony coral Pocillopora damicornis (probably as a cryptic symbiont – Santos et al., 2004). There are scattered reports of Clade B from Acropora species on the Great Barrier Reef (Crabbe and Carlin, 2009).

Figure 5. Phylogenetic radiations of Clade B symbionts from progenitor Clades B1 and B19. These are mostly from the Caribbean, although ‘B’ clades are not particularly uncommon in some Pacific invertebrates (After LaJeunesse, 2005, with additional information from Thornhill et al., 2005).
Clade B has been found to be present in these corals:
Acropora hyacinthus, Aiptasia pallida, Aiptasia pulchella, Astrangia danae, Briareum asbestinium, Calpophyllia natans, Cladocora arbuscula, Dendrogyra cylindrus, Dichocoenia stokesii, Diploria clivosa, Diploria labyrinthiformis, Diploria strigosa, Eunicea aspercula, Eunicea calyculata, Eunicea colombiensis, Eunicea laciniata, Eunicea mammosa, Eunicea pallida, Eunicea species ‘1’ and ‘2’, Eunicea succinea, Eunicea tayrona, Eunicea tourneforti, Eusmilia fastigata, Favia fragum, Gorgonia sp., Gorgonia flabellum, Gorgonia mariae, Gorgonia ventalina, Isophyllastrea rigida, Madracis decactis, Madracis formosa, Madracis mirabilis, Madracis pharensis, Madracis senaria, Manicina aerolata, Meandrina meandrites, Montastrea curta, Montastrea faveolata, Montastrea franksii, Muricea muricata, Muriceopsis flavida, Muriceopsis sp., Muriceopsis urabensis, Plesiastrea verispora, Plexaura flexuosa, Plexaura homomalla, Plexaura kuna, Plexaurella nutans, Porites asteroides, Porites furcata, Porites radians, Pseudoplexaura flagellosa, Pseudoplexaura porosa, Pseudoplexaura sp., Pseudoplexaura wagenarii, Pseudopterogorgia americana, Pseudopterogorgia bipinnata, Pseudopterogorgia elisabethae, Pterogorgia anceps, and Pterogorgia citrina.
Reported Species in Clade “B”
As with Clade ‘A’ zooxanthellae, those of Clade B are relatively resistant to bleaching episodes. Current information suggests this clade is most common in Caribbean octocorals (sea fans, sea whips, etc.), but also present in many (a dozen or more) Atlantic stony coral genera. A subclade (B1) has been found in Hawaiian Aiptasia anemones and stony coral Pocillopora damicornis (probably as a cryptic symbiont Santos et al., 2004).
Symbiodinium bermudense. A symbiont of the ‘pest’ anemone Aiptasia pallida. This species apparently produces MAAs under ‘proper’ conditions (Banaszak et al., 2006).
Symbiodinium californium. This species does not produce mycosporine-like amino acids in culture (in Shick et al., 2002), but other evidence suggests S. californium can perhaps do so under conditions of high light and/or UV intensities. It is found within the Anthopleura elegantissima anemone. S. californium is sometimes listed as Clade “E” (Santos et al., 2001). S. californium is intolerant of colder water hence its distribution is in warmer waters (such as the southern California coast where temperatures are in the range of ~14.5-19°C, or 58.1-66.2°F). Secord and Muller-Parker (2005) found that S. californium is tolerant of high light intensity (PAR, or Photosynthetically Active Radiation) and photosynthetic saturation was not achieved at 540 µmole photons·m²·sec. The compensation point for these algae was about 73 µmoll photons·m²·sec.
Symbiodinium muscatinei. Also called Clade B4. This species has been described as found in tissues of the temperate anemone Anthopleura elegantissima. It is thought that this species does not produce ‘UV sunscreens’ (mycosporine-like amino acids, Shick et al., 2002), but instead acquires them through diet. S. muscatinei is sometimes listed as Clade “E.” (Santos et al., 2001). Secord and Muller-Parker (2005) found that S. muscatinei and S. californium are tolerant of high light intensity (PAR, or Photosynthetically Active Radiation) and photosynthetic saturation was not achieved at 540 µmole photons·m²·sec. The compensation point for these algae was about 73 µmoll photons·m²·sec. Symbiodinium muscatinei is found in cooler waters, generally along the Pacific Northwest coastline where water temperatures are within the range of ~8-15°C or 46.4-59°F.
Symbiodinium pulchrorum. Found in the Hawaiian anemone Aiptasia. Iglesias-Prieto and Trench (1997) report S. pulchrorum has a high photoacclimatory capability (their experiment used 40 µmol·m²·sec as the sub-saturating intensity, and 250 µmol·m²·sec as the super-saturating light intensity). Banaszak (2000) did not find this species to synthesize MAAs, although subsequent research found the level of ultraviolet radiation was insufficient for MAA production (Banaszak, 2006). As a footnote to these observations, I have noticed that Aiptasia anemones do not fare well under high light intensity in outdoor tanks exposed to natural sunlight – they retract into small blobs, probably in an effort to self-shade their zooxanthellae from high PPFD (600 µmol·m²·sec and higher) and/or UV radiation.
Summary for Clade B zooxanthellae species: Synthesis of UV protectants (mycosporine-like amino acids) seems dependent upon environmental conditions (though this is open to debate). Clade B also seems relatively tolerant of higher light intensities.
B Clades and Sub-Clades
B1 (based on ITS2 analysis) orB184 (based on chloroplast genotype)
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean, Hawaii, Gulf of California, New Zealand
- Host Species: Common to many Caribbean invertebrates, including the aquarium pest anemone Aiptasia (from Hawaii, LaJeunesse, 2001), soft coral Capnella (Wicks et al., 2010b), the sea fan Gorgonia ventalina (Kirk et al., 2005), Oculina diffusa (western Atlantic, LaJeunesse, 2001), Caribbean stony coral Diploria clivosa (Banaszak et al., 2006), Diploria strigosa, Favia fragum, the ‘rose’ coral Manicina areolata, Montastrea annularis (LaJeunesse, 2002), the stony coral Pocillopora damicornis in Hawaii, Pocillopora spp. in the Gulf of California, and others, including Pseudopterogorgia bipinnata and various ‘pesky’ anemones (Caribbean Aiptasia spp.).
- Comments: Hennige et. al. (2006) report Clade B1 is tolerant of low temperatures. It was found to be sensitive to a ‘high’ temperature of 26º C (78.8º F), while Robinson and Warner (2006) report thermally-sensitive B1 demonstrated severe decreases in photosynthetic activity when exposed to ‘high’ light and a temperature of 32º C (89.6º F). Suggett et al. (2008) report that clade B1 produces increased amounts of hydrogen peroxide when exposed to temperature of 32º C (89.6º F) – H 2O2 is destructive to living tissues and can be de-toxified by specific enzymes. In the same vein, Gorgonia ventalina specimens from Florida contained less zooxanthellae when exposed to a temperature of 30.5ºC (86.9ºF), or when the host was infected with the fungus Aspergillus sydowii. However, the sea fans retained the same clade throughout the experimental procedures and did not ‘switch’ symbionts. The tolerance of this clade to low temperature is not unlimited: Thornhill et al., 2008, found B1 zooxanthellae lowered their chlorophyll content when exposed to very low temperature (10.5°C – 50.9°F), and did not recover within 3 weeks of exposure (the end of the experimental period).
Clade B1 is equivalent to clade ‘B184’ (based on analysis of the 23S-rDNA; Kirk et al., 2005).
B1 is an opportunistic clade, possessing characteristics allowing it to thrive after periods of environment disturbance (such as coral bleaching due to low water temperatures; LaJeunesse et al., 2010).
B1a
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: Plexaura homomalla and Plexaurella nutans.
- Comments: Closely related to clade B1. LaJeunesse, 2004.
B1b
- Reported Depth Range: 2.5 meters (8 feet)
- Reported Geographical Range: Mexican Caribbean
- Host Species: Plexaura flexuosa
- Comments: LaJeunesse, 2002
B1c
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: Caribbean corals
- Comments: Closely related to clade B1. LaJeunesse, 2004.
B1d
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: Caribbean corals
- Comments: Closely related to clade B1. LaJeunesse, 2004.
B1e
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: Caribbean corals
- Comments: Closely related to clade B1. LaJeunesse, 2004.
B1g
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: Caribbean corals
- Comments: Closely related to clade B1. LaJeunesse, 2004.
B1i
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: Caribbean corals
- Comments: Closely related to clade B1. LaJeunesse, 2004.
B1j
- Reported Depth Range: <5 meters ( feet)
- Reported Geographical Range: Barbados, Caribbean
- Host Species: Montastrea annularis
- Comments: LaJeunesse et al., 2009.
B2
- Reported Depth Range: 3 – 21.3 meters (10 feet to 70 feet)
- Reported Geographical Range: Caribbean, US eastern seaboard (Georgia coast northward to Rhode Island, USA) and US Gulf coast (roughly Tampa, Florida westward to extreme southern Texas coast).
- Host Species: Plexaura flexuosa, and stony corals Astrangia poculata, Montastraea faveolata, Oculina arbuscula, andOculina diffusa.
- Comments: Descended from Clade B19. LaJeunesse, 2004. Considered to be tolerant of low light and low temperature and recovers quickly after exposure to temperatures as low as 10.5°C (50.9°F; Thornhill et al., 2008).
B2.1
- Reported Depth Range: ?
- Reported Geographical Range: Bermuda
- Host Species: Oculina diffusa
- Comments: LaJeunesse, 2001.
B3
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: Caribbean ‘jellyfish’ Dichotoma.
- Comments: LaJeunesse, 2001.
B4 (Symbiodinium muscatinei)
- Reported Depth Range: ?
- Reported Geographical Range: NW Pacific
- Host Species: Anemone Anthopleura elegantissima
- Comments: B4 is Symbiodinium muscatinei, reportedly found in only temperate/cold waters. (LaJeunesse, 2001).
B5
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: Siderastraea radians.
- Comments: A specialist zooxanthellae found only in the Caribbean coral Siderastraea radians (LaJeunesse, 2004).
B5a
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Siderastrea siderea
- Comments: Specialist zooxanthellae clade found only in Siderastrea (Thornhill et al., 2006).
B6
- Reported Depth Range: ?
- Reported Geographical Range: Western Caribbean
- Host Species: Colpophyllia natans from the western Caribbean.
- Comments: Descended from Clade B19. (LaJeunesse, 2004).
B7
- Reported Depth Range: ?
- Reported Geographical Range: Southern and western Caribbean
- Host Species: Madracis decactis (Family Pocilloporidae)
- Reference: LaJeunesse, 2004.
B8
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: Gorgonian Pseudoplexaura flexuosa
- Comments: Closely related to clade B1 (LaJeunesse, 2004).
B9
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: The stony coral Colpophyllia natans and gorgonian Eunicea mammosa.
- Comments: Descended from Clade B19 (LaJeunesse, 2004).
B10
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: Montastrea annularis, M. faveolata and M. franksi
- Comments: A specialist zooxanthella clade from Caribbean corals Montastrea annularis, M. faveolata and M. franksi (Thornhill et al., 2005). Closely related to clade B1 (LaJeunesse, 2004).
B11
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: ‘Caribbean corals’
- Comments: Closely related to clade B1. (LaJeunesse, 2004).
B12
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: ‘Caribbean corals’
- Comments: Closely related to clade B1. (LaJeunesse, 2004).
B13
- Reported Depth Range: ?
- Reported Geographical Range: Southern Caribbean
- Host Species: Madracis spp.
- Comments: A specialist clade ( LaJeunesse, 2004).
B13a
- Reported Depth Range: ?
- Reported Geographical Range: Northeast Caribbean
- Host Species: Madracis spp.
- Comments: A specialist clade (LaJeunesse, 2004).
B14
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: ‘Caribbean corals’
- Reference: LaJeunesse, 2004.
B16
- Reported Depth Range: ?
- Reported Geographical Range:
- Host Species: ‘Caribbean corals’
- Comments: Closely related to clade B1. LaJeunesse, 2004
B17
- Reported Depth Range: ?
- Reported Geographical Range: Belize and Caribbean
- Host Species: Montastraea faveolata
- Comments: Closely related to clade B1. LaJeunesse, 2004.
B19
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: Briareum
- Comments: B19 is believed to be an ancestor to many ‘B’ Clades. It has been isolated from a newly settled polyp of the Caribbean ‘soft coral’ Briareum, LaJeunesse, 2005.
B19a
- Reported Depth Range: ?
- Reported Geographical Range: Northeast Caribbean
- Host Species: The stony coral Colpophyllia
- Comments: Descended from Clade B19. LaJeunesse, 2004.
B19b
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: ‘Caribbean corals’
- Comments: Closely related to clade B19. LaJeunesse, 2004.
B20
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: ‘Caribbean corals’
- Comments: Closely related to clade B1. LaJeunesse, 2004.
B21
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: Caribbean corals
- Comments: Descended from Clade B19. LaJeunesse, 2004.
B22
- Reported Depth Range:
- Reported Geographical Range:
- Host Species: Colpophyllia
- Comments: Descended from Clade B19. LaJeunesse, 2004.
B23
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: Caribbean corals
- Comments: Descended from Clade B19. LaJeunesse, 2004.
B24
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Host Species: Caribbean corals
- Comments: Closely related to clade B1- LaJeunesse, 2004.
B25
- Reported Depth Range: ?
- Reported Geographical Range: Florida
- Host Species: Briareum
- Comments: Isolated from a newly settled polyp of the ‘soft coral’ Briareum (LaJeunesse, 2005).
B26
- Reported Depth Range: ?
- Reported Geographical Range: Panamanian Caribbean
- Host Species: gorgonian Plexaura kuna
- Note: Descended from Clade B19 (LaJeunesse, 2004).
B170
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Reported Hosts: Caribbean stony coral Porites divaricata
- Comment: The numerical portion of the clade ID is based on the length (bp) of a variable region in the chloroplast 23S rDNA gene, and not the ITS1 or ITS2 regions used by many researchers. This clade is tolerant of temperatures of at least 26°C (78.8°C). Experimental temperature of 33°C (91.4°F) was used to bleach P. divaricata of its symbionts.
- Reference: Coffroth et al., 2010.
B184
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Reported Hosts: Gorgonian Gorgonia ventalina
- Comment: The numerical portion of the clade ID is based on the length (bp) of a variable region in the chloroplast 23S rDNA gene, and not the ITS1 or ITS2 regions used by many researchers.
- Reference: Coffroth et al., 2010.
B211
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Reported Hosts: Caribbean corals
- Comment: The numerical portion of the clade ID is based on the length (bp) of a variable region in the chloroplast 23S rDNA gene, and not the ITS1 or ITS2 regions used by many researchers.
- Reference : Coffroth et al., 2010.
B223
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Reported Hosts: Caribbean corals
- Comment: The numerical portion of the clade ID is based on the length (bp) of a variable region in the chloroplast 23S rDNA gene, and not the ITS1 or ITS2 regions used by many researchers.
- Reference : Coffroth et al., 2010.
Clade B224
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Reported Hosts: Caribbean corals
- Comment: The numerical portion of the clade ID is based on the length (bp) of a variable region in the chloroplast 23S rDNA gene, and not the ITS1 or ITS2 regions used by many researchers.
- Reference : Coffroth et al., 2010.
Clade C
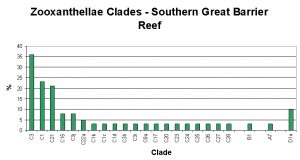
Figure 6. Zooxanthellae clades found in corals of Australia’s southern Great Barrier Reef. After LaJeunesse.
Clade C, as a group, is difficult to characterize, though Atlantic Clade C zooxanthellae are found in deeper water, while bleaching is often noted in Pacific corals containing Clade C symbionts. Generally, most Clade C zooxanthellae/corals inhabit tropical latitudes.
Clade C contains over 130 subclades, and, as a group, is pandemic. It is found in some Caribbean corals, but most often in the Pacific.
Some Clade Cs are thermally-tolerant (C15), others are generalists exhibiting habitation over a broad range of depths (C1, C3 and C21), C8a is found only in deeper waters, C7c is limited to relatively shallow depths and in nature tolerates light intensity up to about 700 µmol·m²·sec. It is easy to see why Tchernov et al., 2004 warn of assuming closely related sister subclades will demonstrate similar traits (light and/or temperature tolerances for example).

Figure 7. Adaptive radiations of Pacific zooxanthellae from an ‘ancestral core’ consisting of Clades C1, C3, C21 and C1c. (After LaJeunesse, 2005 – see that work for more information than this diagram illustrates).
Early literature reports only general types of clades (A, B, C, etc.). The following is a list of those taxa generalized to Clade C. In some cases, the type of symbiont has not been revisited and analyzed by more sophisticated techniques, and it is the only information we have. Those taxa listed simply as Clade C:
Acropora sp., Acropora aspera, Acropora cervicornis, Acropora cuneata, Acropora divaricata, Acropora gemmifera, Acropora glauca, Acropora latistella, Acropora longicyathus, Acropora millepora, Acropora spathulata, Acropora tenuis, Agaricia agaricities, Agaricia danae, Agaricia fragilis, Agaricia humilis, Agaricia lamarcki, Agaricia tenufolia, Alertigorgia orientalis, Alveopora japonica, Anthelia glauca, Briareum asbestinium, Briareum violacea, Cespitularia sp., Cespitularia pachyclados, Cespitularia tuberculoides, Colpophyllia natans, Cyphastrea ocellina, Diploria clivosa, Diploria labyrinthiformis, Diploria strigosa, Entacmaea quadricolor, Erythropodium caribaeorum, Eusmilia fastigata, Favia fragum, Favia matthai, Fungia paumotensis, Fungia scutaria, Gardineroseris planulata, Helipora coerulea, Heteroxenia fuscenscens, Hippopus hippopus, Isophyllastrea rigida, Isophyllia sinuosa, Klyxum sp., Lemnalia, Leptastrea transversa, Leptoseris cucullata, Lobophyllia sp., Lobophytum (described as species 1 and 2), Manicina aerolata, Montastrea annularis, Montastrea cavernosa, Montastrea faveolata, Montastrea franksii, Montipora patula, Montipora verrucosa/capitata, Mycetophyllia danae, Mycetophyllia ferox, Mycetophyllia lamarckiana, Oulophyllia crispa, Pachyseris speciosa, Pachyseris superficialis, Paralemnalia eburnea, Paralemnalia (described as species # 1 and 2), Paralemnalia thyrsoides, Parasicyonis, Pavona cactus, Pavona clavus, Pavona divaricate, Pavona duerdeni, Pavona gigantea, Pavona varians, Plexaurella dichotoma, Plexaura grisea, Plexaura nutans, Plumigorgia schoboti, Pocillopora damicornis, Pocillopora elegans, Pocillopora eydouxi, Pocillopora verrucosa, Porites astreoides (brown and green variants), Porites colonensis, Porites compressa, Porites cylindrica, Porites divaricata, Porites furcate, Porites lobata, Porites panamensis, Porites porites, Porites rus, Psammocora stellata, Psammocora superficialis, Rhytisma fulvum fulvum, Rhytisma (described as species #1 and #2), Sarcophyton glaucum, Sarcophyton trocheliophorum, Sarcophyton (described as species #1 and #2), Scolymia cubensis, Seriatopora hystrix, Siderastrea siderea, Siderastrea stellata, Sinularia sp., Sinularia gardineri, Sinularia leptoclados, Sinularia polydactyla, Sinularia querciformis, Sinularia (described as species #1 and #2), Stephanocoenia intersepta, Stephanocoenia michelinii, Tubipora musica, Xenia (described as species #1 and #2), Xenia farauensis, Xenia microspiculata, Xenia umbellata, and “Xenidae“.

Figure 8. The same diagram as in Figure 7, but with added information. Note that some zooxanthellae clades are found predominantly in some coral genera (colored areas).
As Figure 4 demonstrates, those corals containing Clade C are dominant on Australia’s Great Barrier Reef.
C·
- Reported Depth Range: ?
- Reported Geographical Range: Great Barrier Reef and Indonesia
- Clade C· – This zooxanthella is believed to have co-evolved with Montipora species, but sometimes found in Porites attenuata and Porites cylindrica. Montipora species containing Clade C· include Montipora aequituberculata, M. altasepta, M. angulata, M. cactus, M. capitata, M. crassituberculata, M. danae, M. delicatula, M. digitata, M. gaimardi, M. hispida, M. hoffmeisteri, M. mollis, M. peltiformis, M. spongodes,
- M. stellata, M. turtlensis, M. undata, and M. verrucosa (van Oppen et al., 2004). This clade is presently known to be distributed from Indonesia southward to the Great Barrier Reef. One has to wonder if this clade has high-fidelity to Montipora spp. and is one of those listed in LaJeunesse’s more-or-less concurrent paper (namely Clades C17, C26a, C27, C30, C31, C31a, C31b, C32, C58 and C73). van Oppen’s IDs are based on ITS1 sequences (while LaJeunesse’s – and many others’- are based on ITS2).
C+
- Reported Depth Range: ?
- Reported Geographical Range: Pacific
- Host Species: Montipora danae
- Comments: Based on an ITS 1 sequences (most are based on ITS 2) – van Oppen 2004.
C’
- Reported Depth Range: 0-14 meters (0 – 46 feet)
- Reported Geographical Range: Panamanian Caribbean
- Host Species: Montastrea franksi
- Comments: A positive correlation has been established between this clade/coral and high sedimentation rates (Gareen et al., 2006).
C-Lep
- Reported Depth Range: Shallow tide pool
- Reported Geographical Range: American Samoa
- Host Species: Pavona cactus, Leptoria phygra
- Comments: These researchers (Oliver and Palumbi, 2011) analyzed
- chloroplast 23 s rDNA.
Cmp
- Reported Depth Range: 10 -20 meters (33 – 65 feet)
- Reported Geographical Range: Hawai’i
- Host Species: Montipora patula
- Comments: Thornhill, 2003
Cpav
- Reported Depth Range: 2 -20 meters (7 – 65 feet)
- Reported Geographical Range: Hawai’i
- Host Species: Pavona duerdeni, Pavona varians
- Comments: Thornhill, 2003
Cpav
- Reported Depth Range: Shallow tide pool
- Reported Geographical Range: American Samoa
- Host Species: Pavona cactus, Leptoria phygra
- Comments: Apparently different from Thornhill’s Cpav (above), as these researchers (Oliver and Palumbi, 2011) analyzed chloroplast 23 s rDNA.
Cpr
- Reported Depth Range: 10 -20 meters (33 – 67 feet)
- Reported Geographical Range: Hawai’i
- Host Species: Porites rus
- Comments: Thornhill, 2003
C1pd
- Reported Depth Range: 1 – 2 meters (3 -7 feet)
- Reported Geographical Range: Hawai’i
- Host Species: Pocillopora damicornis
- Comments: Thornhill, 2003
C1unk
- Reported Depth Range: 3-6 meters (10-20 feet)
- Reported Geographical Range: Heron Island, southern Great Barrier Reef
- Host Species: Stylophora pistillata
- Comments: Found in a S. pistillata colony also containing clades C35 and C35a. This coral colony experienced severe bleaching when water temperature reached 28.1°C (82.6°C). C1unk differs from C1b by only 1 bp (base pair – a ‘rung’ of the DNA ladder). Sampayo et al., 2008.
C1zoan
- Reported Depth Range: 1 – 2 meters (3 -7 feet)
- Reported Geographical Range: Hawai’i
- Host Species: Protopalythoa
- Comments: Thornhill, 2003
C1
- Symbiodinium goreaui. Originally found within Ragactis lucida (in Trench, 1996) and expanded by LaJeunesse et al., 2003 to the pandemic generalist zooxanthellae Clade C1.
- Reported Depth Range: 0-20 meters (0-65 feet)
- Geographical Range: Pandemic-Bahamas, Japan, Korea, Great Barrier Reef, Atlantic and Mexican Caribbean, Taiwan, Hawaii, Indonesia
- Host Species: Acropora abrolhosensis, A. cervicornis, A. cuneata, A. divaricata, A. donei, A. humilis, A. hyacinthus, A. longicyathus, A. millepora, A. nobilis, A. palifera, A. sarmentosa, A. secale, A. tenuis, A. verweyi, Alveopora fenestrate, Astreopora sp., Astreopora myriophthalma, Bartholomea sp., Bartholomea annulata, Caulastrea chalcidicum, Condylactis, ‘Corallimorpharia’, Coscinarea sp., Coscinarea columna, C. wellsi, Cycloseris vaughani, Cyphastrea sp., Cyphastrea chalcidicum, C. decadia, C. japonica, C. microphthalma, C. serailia, Discosoma sp., D. carlgreni, D. sanctithomae, Echinophyllia, E. echinoporoides, E. orpheensis, E. lamellosa, Eunicea, Euphyllia sp., E. ancora, E. divisa, E. glabrescens, Favia sp., F. favus, F. pallida, F. speciosa, Favites abdita, Fungia sp., F. crassa, F. echinata, F. fungites, F. granulosa, F. scutaria, Galaxea sp., Galaxea astreata, Goniastrea sp., Goniastrea australensis, G. favulus, G. pectinata, G. rectiformis, Goniopora sp., G. columna, G. djiboutiensis, G. lobata, G. minor, G. tenuidens, Heliopora coerulea, Herpolitha sp., H. weberi, Hydnophora sp., H. exesa, H. rigida, Icilogorgia, Lebrunia sp., Lebrunia danae, Leptastrea sp., Leptastrea incrustans, L. pruinosa, L. purpurea, L. phrygia, Leptoseris incrustans, L. yabei, Linuche sp., L. unguiculata, Lithophyllon undulatum, Lobophytum sp., Merulina sp., Merulina ampliata, M. scrabicula, Millepora sp., Millepora exaesa, Montastrea curta, M. valenciennesi, Montipora sp., Montipora aequituberculata, M. cactus, M. confuse, M. digitata, M. efflorescens, M. hispida, M. spongodes, M. undata, Mycedium sp., Mycedium elephantotus, Pachyseris sp., Pachyseris rugosa, Palauastrea sp., Palauastrea ramosa, Palythoa sp., Palythoa caribaeorum, Pavona desucata, P. duerdeni, P. frondifera, P. varians, Pavona venosa, Plerogyra sp.,Plerogyra sinuosa, Plesiastrea verispora, Plexaura, Plumigorgia sp., Pocillopora damicornis, Polyphyllia sp., Polyphyllia talpina, Porites sp., P. cylindrica, P. divaricata, P. lutea, P. solida, Psammocora sp., P. contigua, P. digitata, P. profundacella, Pseudosiderastrea tayamai, Pteraeolidia, Rhodactis sp.,Rhodactis (Heteractis) lucida, Rhystima sp., Rumphella sp., Sarcophyton sp., Scolymia sp., Scolymia australis, Siderastrea sp., Siderastrea siderea, Sinularia sp., Stylocoeniella guentheri, Stylophora sp., Stylophora pistillata, Tridacna sp., Tridacna derasa, T. gigas, T. maxima, Turbinaria sp., Turbinaria frondens, Turbinaria mesenteria, T. stellulata, and Zoanthus sp..
C1:1
- Reported Depth Range: 2-6m (6-20 feet)
- Reported Geographical Range: Central Great Barrier Reef
- Host Species: Pinnigorgia flava
- Comments: Goulet et al., 2008
C1:1a
- Reported Depth Range: 2-6m (6-20 feet)
- Reported Geographical Range: Central Great Barrier Reef
- Host Species: Briareum, Lemnalia, Paralemnalia spp.
- Comments: Goulet et al., 2008
C1:2
- Reported Depth Range: 2-6m (~7 – 20 feet)
- Reported Geographical Range: Central Great Barrier Reef
- Host Species: Briareum sp., Lemnalia sp., Paralemnalia digitiformis, and Paralemnalia thyrsoides
- Comments: Goulet et al., 2008
C1:3a
- Reported Depth Range: 2-6m (~7 – 20 feet)
- Reported Geographical Range: Central Great Barrier Reef
- Host Species: Efflatounaria, Lobophytum compactum, Sarcophyton sp., Sinularia flexibilis, and Sinularia polydactyla
- Comments: Goulet and Coffroth, 2004; Goulet et al., 2008
C1a
- Reported Depth Range: 7-25 meters (23 feet – 82 feet)
- Reported Geographical Range: Caribbean, Pacific
- Host Species: Fungia scutaria, Porites asteroides, and Porites colonensis.
- Comments: Believed to have evolved from Clade C1. LaJeunesse, 2005. A Fungia scutaria transplanted from the Pacific to Jamaica by pioneer researcher Thomas Goreau was found to have retained Clade C1a decades after the transfer. Although valuable information was obtained from these transferred corals, some scientists considered the Fungia specimens to be invasive, and a search-and-destroy mission eliminated (or greatly reduced) their population.
C1b
- Reported Depth Range: 3 – 17 meters (10 – 56 feet)
- Reported Geographical Range: Western Pacific
- Host Species: Leptastrea sp., Leptastrea purpurea, Pavona sp., Pavona superficialis, Pavona varians, Tubipora sp. and Tubipora musica.
- Comments: Believed to have evolved from Clade C1. LaJeunesse, 2005.
C1bb
- Reported Depth Range: 15m (49 feet)
- Reported Geographical Range: Lord Howe Island, Australia
- Host Species: Stylophora pistillata
- Comments: Wicks et al., 2010b
C1bc
- Reported Depth Range: 1 – 6 meters (~3 – 20 feet)
- Reported Geographical Range: Eastern Pacific; Gulf of California southward to the Gulf of Panama
- Host Species: Pocillopora species.
- Comments: Believed to have evolved from Clade C1. Thermally sensitive to low water temperatures. LaJeunesse et al., 2003; 2010.
C1b-f
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Pavona cactus, Pavona decussata
- Comments: Aquarium corals, Smith et al., 2009
C1c
- Reported Depth Range: 1 – 25 meters (3 – 82 feet)
- Reported Geographical Range: Hawai’i, Okinawa, north and south Great Barrier Reef, Eastern Pacific
- Host Species: Acropora cuneata, Pocillopora ligulata, Pocillopora meandrina, Pocillopora damicornis, Pocillopora eydouxi, a Pocillopora hybrid, Pocillopora verrucosa, Pavona sp., Pavona gigantean and the soft coral Sinularia.
- Comments: Clade C1c (along with C1, C3, C21, C3d, and C45) is believed to be an ancestral type from which other clades evolved (LaJeunesse, 2005). Found in Pocillopora specimens from the western, central and south Pacific (Okinawa and GBR), a Pavona specimen and an unidentified soft coral from the eastern Pacific. PAM fluorometry work found onset of photosaturation at 275 µmol photons·m²·sec (~5,500 lux) and onset of photoinhibition at ~425 µmol photons·m²·sec (~21,250 lux) in a shallow-water Hawaiian Pocillopora meandrina specimen (ITS 2 analysis by Smith et al., 2009; PAM fluorometry by Riddle, 2007: http://www.advancedaquarist.com/2007/3/aafeature1/ Ulstrap et al., 2006 report a Pocillopora damicornis specimen possibly containing C1c (or C1j, a subclade of C1) showing photoinactivation (a decrease in photosynthetic activity) at lower irradiance levels in shaded portions of the colony than in those parts exposed to direct light. This suggests these zooxanthellae have an ability to fine tune light absorption according to their exposure, or perhaps that these corals contain a yet unreported zooxanthellae clade that inhabits only shaded portions of the coral colony. Results of testing of ITS1 and ITS2 regions of DNA will both identify the clade as C1c.
C1d
- Reported Depth Range: 1-5 meters (~3-16 feet)
- Reported Geographical Range: Hawaii; Gulf of California; Panama (Pacific); Clipperton Atoll
- Host Species: Pocillopora spp., Pocillopora damicornis
- Comments: Found in Pacific Pocillopora spp. (there are no Pocillopora species in the Atlantic; LaJeunesse 2004). C1d is a variant of C42 (which is thought to be a variant of Clade C1c, itself a variant of Clade C1, LaJeunesse, 2005). It is currently believed to be restricted in distribution compared to Clade C1c.
C1d-t
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Pocillopora damicornis
- Comments: Aquarium coral. Smith et al., 2009
C1e
- Reported Depth Range: 2-15 meters (7-49 feet)
- Reported Geographical Range: Hawaii
- Host Species: Cyphastrea sp., Fungia sp., Leptastrea sp., Psammocora sp.
- Reference: LaJeunesse et al., 2004.
C1e-e
- Reported Depth Range: ?
- Reported Geographical Range: Gulf of California southward to Gulf of Panama
- Host Species: Pocillopora sp.
- Reference: LaJeunesse et al., 2009.
C1f
- Reported Depth Range: 2-15 meters (7-65 feet)
- Reported Geographical Range: Hawaii, eastern Pacific
- Host Species: Found in central Pacific (Hawaiian) stony corals Cyphastrea, Fungia, Leptastrea, and eastern/central Pacific Psammocora spp. Collection depths ranged from 2-15m (LaJeunesse 2004). Believed to have evolved from Clade C1 (LaJeunesse, 2005).
C1g
- Reported Depth Range: 20 meters (65 feet)
- Reported Geographical Range: Hawaii
- Host Species: Pocillopora sp., Pocillopora ligulata
- Comments: C1g is believed to have evolved from Clade C1, and is possibly endemic to Hawai’i. LaJeunesse, 2004.
C1h
- Reported Depth Range: 2 meters (7 feet)
- Reported Geographical Range: Hawai’i
- Host Species: Pocillopora sp.
- Comments: Believed to have evolved from Clade C1. LaJeunesse, 2005.
C1ha
- Reported Depth Range: 2-20 meters (7 – 65 feet)
- Reported Geographical Range: Hawai’i
- Host Species: Fungia scutaria, Leptastrea bottae
- Reference: Thornhill, 2003
C1hb
- Reported Depth Range: 10-20 meters (33 – 65 feet)
- Reported Geographical Range: Hawai’i
- Host Species: Leptastrea purpurea, Psammocora haimeana
- Reference: Thornhill, 2003
C1hc
- Reported Depth Range: 2 meters (7 feet)
- Reported Geographical Range: Hawai’i
- Host Species: Cyphastrea ocellina, Fungia scutaria, Psammocora sp.
- Reference: Thornhill, 2003
C1i
- Reported Depth Range: 8 – 25 meters (26 – 82 feet)
- Reported Geographical Range: Caribbean and Great Barrier Reef
- Host Species: Cyphastrea serailia (GBR) and Porites asteroids (Caribbean)
- Comments: Believed to have evolved from Clade C1. LaJeunesse, 2005.
C1j
- Reported Depth Range: 1 – 17 meters (3 – 56 feet)
- Reported Geographical Range: Great Barrier Reef
- Host Species: Pocillopora damicornis
- Comments: Believed to have evolved from Clade C1. LaJeunesse, 2004
C1k
- Reported Depth Range: 10 – 17 meters (33 – 56 feet)
- Reported Geographical Range: Western Pacific, Great Barrier Reef
- Host Species: Xenia sp.
- Comments: A variant of C1c (which likely evolved from Clade C1). LaJeunesse, 2005.
C1L
- Reported Depth Range: 7 meters (23 feet)
- Reported Geographical Range: Eastern Pacific
- Host Species: Protopalythoa
- Reference: LaJeunesse et al., 2003
C1m
- Reported Depth Range: 7 meters (23 feet)
- Reported Geographical Range: Eastern Pacific
- Host Species: Protopalythoa
- Reference: LaJeunesse et al., 2003
C1n
- Reported Depth Range: ?
- Reported Geographical Range: Red Sea
- Host Species: The stony coral Turbinaria sp.
- Reference: Barneah et al., 2007.
C1q
- Reported Depth Range: 2-6m (~7 – 20 feet)
- Reported Geographical Range: Central Great Barrier Reef
- Host Species: Cespitularia
- Comments: Goulet et al., 2008
C1z
- Reported Depth Range: 5-10m (~16 – 33 feet)
- Reported Geographical Range: Kermadec Islands, New Zealand
- Host Species: Sinularia sp., Hydnophora pilosa
- Comments: Wicks et al., 2010b
C2
- Reported Depth Range: 0 – 17 meters (0 – 56 feet)
- Reported Geographical Range: Pandemic: Caribbean, Great Barrier Reef, Indonesia, Taiwan
- Host Species: Found largely in Pacific Acroporidae (Acropora, Pocillopora, Montipora) including Acropora aspera, Acropora cerealis (GBR, Van Oppen, 2001), Acropora cervicornis (Caribbean 2.0-17.0m, Baker et al., 1997), Acropora cuneata (Van Oppen et al., 2005), Acropora florida, Acropora gemmifera, Acropora intermedia, Acropora longicyathus, Acropora loripes, Acropora millepora, Acropora nastua, Acropora spathulata, Acropora tenuis, Acropora valida (GBR, Van Oppen, 2001), Goniastrea rectiformis (Van Oppen, 2005), zooxanthellae collected and cultured from the clam Hippopus (LaJeunesse, 2003), Montipora aequituberculata, Montipora capricornis, Montipora danae, Montipora florida (from Indonesia, Van Oppen, 2005 based on ITS2 fingerprint), Pavona varians (Van Oppen, 2005), and Pocillopora damicornis (two locations in Taiwan, 0-5.0m, Chen et al., 2005).
- Comments: Iglesias-Prieto and Trench (1997) believe clade C2 has good photoacclimatory abilities. However, Berkelmans and van Oppen (2006) state C2 is thermally sensitive to elevated temperatures. Thornhill et al., 2008, found C2 zooxanthellae lowered their chlorophyll content when exposed to very low temperature (10.5°C – 50.9°F), and did not recover within 3 weeks of exposure (the end of the experimental period).
C2*
- Reported Depth Range: 2 – 4 meters (7 – 15 feet)
- Reported Geographical Range: Davies Reef, Great Barrier Reef
- Host Species: Acropora millepora
- Comments: Berkelmans and van Oppen (2006) state the ITS1 rDNA in C2* differs from than in C2 zooxanthellae, and is less thermally sensitive than C2.
C3
- Reported Depth Range: 0-90 meters (0-294 feet)
- Reported Geographical Range: Bahamas, Belize, Okinawa, Taiwan, Great Barrier Reef, Hawai’i, Mexican Caribbean, U.S. Virgin Islands, Kenya
- Host Species: This clade is a pandemic generalist zooxanthella, reports seem to indicate this clade is remarkably adaptable over a bathymetric range of 0.3 – 90m (1-294 feet deep, and perhaps even deeper), and it is generally assumed to be adapted to deeper water environments. LaJeunesse, 2002 reports that C3 – along with C3a – were dominant in those corals situated deeper than 5m in waters of the Yucatan (Mexico). Clade C3 (along with C1, C21, C3d, C1c and C45) is believed to be an ancestral type from which other clades evolved (LaJeunesse, 2004). Those corals infected with Clade C3 include Acanthastrea and Acropora (1.0- 90m, LaJeunesse et. al., 2003), Acropora abretinoides, Acropora aculeus (GBR, Van Oppen, 2001), Acropora cuneata (Lord Howe Island, GBR; Wicks et al., 2010), Acropora digitifera, Acropora gemmifera, Acropora glauca, Acropora humilis (Taiwan, 3-5m, Chen, 2005), Acropora hyacinthus (two locations in Taiwan, ranging in depths of 3-10m, Chen, 2005), Acropora intermedia (two locations in Taiwan, ranging in depths of 3-10m, Chen, 2005), Acropora latistella (8-10m, Taiwan, Chen, 2005), Acropora latistella (Van Oppen, 2005), Acropora millepora (GBR, Van Oppen, 2001), Acropora muricata (formosa) (1.0-5.0, Taiwan, Chen, 2005), Acropora palifera (Taiwan, 0-3.0m, Chen, 2005), Acropora pulchra (Taiwan, 5-8m, Chen, 2005), Acropora tenuis (Chen, 2005; Taiwan, 5-8m), Acropora valida (Chen, 2005, Taiwan, 5-8m), Acropora valida (Van Oppen, 2001), Acropora yongei (Chen 2005, Taiwan, 3-5m), Acropora yongei (Solomon Islands), the Hawaiian anemone Boloceroides mcmurrichi, Caulastrea, Cyphastrea, Cyphastrea serailia, Diploastrea, Discosoma sp. (Smith et al., 2009), Echinopora, Caribbean Erythropodia, Favia, Favites (LaJeunesse, 2003), Favites abdita, Galaxea fascicularis (Taiwan, 3-5m, Chen, 2005), Goniastrea sp., Goniastrea favulus, Atlantic Gorgonia, Hydnophora pilosa, the Pacific soft coral Isis, Caribbean Isophyllastrea, Pacific Leptoria, Leptoseris (known to inhabit depths of 90m and more), Lobophyllia, Merulina, Atlantic and Pacific Montastraea (Warner et al., 2006), Montastrea curta, Atlantic and Hawaiian Palythoa spp. (LaJeunesse et al., 2003; LaJeunesse, 2004), Platygyra (LaJeunesse et al., 2003), Kenyan Porites cylindrica (Visram and Douglas, 2006), Porites heronensis, Seriatopora (LaJeunesse et al., 2003), Seriatopora hystrix, Siderastrea (Caribbean, LaJeunesse et al., 2003), Siderastrea intersepta (Belize, 8-25m, Warner, 2006), Siderastrea siderea (Belize, 2-25m, Warner, 2006), New Zealand Sinularia, Caribbean Stephanocoenia, Pacific Symphyllia, Turbinaria frondens (New Zealand; Wicks et al., 2010b), and Caribbean Viatrix (LaJeunesse et al., 2003).
C3a
- Reported Depth Range: 1-25 meters (3 -82 feet)
- Reported Geographical Range: Belize to the Bahamas
- Host Species: This specialist clade is reported from Caribbean Agaricia specimens, including A. agaricities (both brown and yellow; LaJeunesse et al., 2003; Thornhill et al., 2006), A. fragilis, A. humilis, A. lamarcki (Warner, 2006) and A. tenufolia.
C3b
- Reported Depth Range: 10 -25 meters (33 – 82 feet)
- Reported Geographical Range: Belize and northern Caribbean
- Host Species: This clade seems to also be a specialist, found only in Caribbean Agaricia agaricities and A. lamarki.
C3c
- Reported Depth Range: 3 -12 meters (10 – 39 feet)
- Reported Geographical Range: Mexican Caribbean; Bahamas
- Host Species: Agaricia agaricites, Isophyllia sinuosa, Mycetophyllia danaana, Mycetophyllia lamarckiana, Mycetophyllia sp., Ricordea florida.
C3d
- Reported Depth Range: 2-3 meters (7-10 feet)
- Reported Geographical Range: Western Caribbean
- Host Species: Montastrea sp., Montastrea cavernosa
- Comments: Clade C3d (along with C1, C3, C21, C1c and C45) is believed to be an ancestral type from which other clades evolved (LaJeunesse, 2004).
C3e
- Reported Depth Range: 2.5 -25 meters (8 – 82 feet)
- Reported Geographical Range: Belize; Western Caribbean
- Host Species: Montastraea cavernosa
- Reference: Warner, 2006
C3f
- Reported Depth Range: 2 – 3 meters (7 – 10 feet)
- Reported Geographical Range: Caribbean
- Host Species: Montastrea sp.
C3g
- Reported Depth Range: 8 -25 meters (26 – 82 feet)
- Reported Geographical Range: Caribbean
- Host Species: Montastrea sp., Montastrea cavernosa
- References: LaJeunesse et. al., 2003; Warner et al., 2006
C3gg
- Reported Depth Range: 15m (49 feet)
- Reported Geographical Range: Lord Howe Island, Australia
- Host Species: Stylophora pistillata
- References: Wicks et al., 2010b
C3h
- Reported Depth Range: 1 – 20 meters (3 – 65 feet)
- Reported Geographical Range: Northern and Central Great Barrier Reef
- Host Species include Acropora aculeus, Barabattoia sp., Caulastrea sp., Caulastrea furcata, Cynarina sp., Cynarina lacrymalis, Echinophyllia sp., Echinophyllia aspera, Echinophyllia echinata, Echinophyllia orpheensis, Echinopora sp., Echinopora gemmacea, Echinopora hirsutissima, Favites sp., Favites abdita, Fungia sp., Fungia echinata, Fungia fungites, Fungia granulosa, Fungia horrida, Fungia paumotensis, Gardineroseris sp., Goniastrea sp., Goniastrea pectinata, Heliofungia actiniformis, Hydnophora sp., Hydnophora exesa, Leptastrea pruinosa, Leptoseris yabei, Lithophyllia sp., Lithophyllon undulatum, Lobophyllia sp., Lobophyllia hemprichii, Mycedium sp., Mycedium elephantotus, Oulophyllia sp., Oulophyllia crispa, Oxypora sp., Oxypora glabra, Oxypora lacera, Pachyseris sp., Pachyseris rugosa, Pachyseris speciosa, Pavona sp., Pavona explanulata, Pavona maldivensis, Pectinia sp., Pectin lactuca, Platygyra sp., Platygyra daedalei, Platygyra pinni, Platygyra ryukyuensis, Podobacia sp., Polyphyllia sp., Polyphyllia talpina, Sandalolitha sp., Sandalolitha robusta, Turbinaria frondens, and Turbinaria sp..
- Comments: Clade C3h could be considered a generalist clade as it inhabits at least 24 coral genera over a broad range of depths (LaJeunesse et. al., 2003). Common in corals of the Australia’s GBR.
C3ha
- Reported Depth Range: 1 – 17 meters (3 – 56 feet)
- Reported Geographical Range: Central Great Barrier Reef, Australia
- Host Species: Gardineroseris, Lobophyllia and Oxypora spp.
- Comments: A sub-clade of C3h. ( LaJeunesse, 2003)
C3i
- Reported Depth Range: 1 – 10 meters (3 – 33 feet)
- Reported Geographical Range: Western Pacific, Great Barrier Reef
- Host Species: Acropora sp., Acropora cerealis, Acropora cuneata, Acropora florida, Acropora gemmifera, Acropora humilis, Acropora nastua, Acropora nobilis, Acropora tenuis, and Acropora valida.
- Comments: Believed to have evolved from Clade C1. LaJeunesse et. al., 2003. Reported from Acropora specimens only.
C3j
- Reported Depth Range: 2-4 meters (7 – 13 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef; Kermadec Islands, New Zealand
- Host Species: Found (so far) in only soft coral genera Lobophytum, Sarcophyton and Sinularia spp.
- Reference: LaJeunesse, 2004
C3k
- Reported Depth Range: 1 – 17 meters (3 – 56 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef; New Zealand
- Host Species: Acropora spp., Acropora cuneata, Acropora digitifera, Acropora divaricata, Acropora florida, Acropora humilis, Acropora hyacinthus, Acropora millepora, Acropora monticulosa, Acropora nastua, Acropora nobilis, Acropora palifera, Acropora valida.
- Comments: Clade found in Acropora species only.
C3L
- Reported Depth Range: 25 meters (82 feet)
- Reported Geographical Range: Caribbean
- Host Species: Colpophyllia and Diploria spp.
- Comments: LaJeunesse et al., 2003
C3m
- Reported Depth Range: 0.5 – 1 meter (1.5 – 3 feet)
- Reported Geographical Range: Hawaii
- Host Species: Hawaiian Protopalythoa.
- Comments: Believed to have evolved from Clade C1 and C3, making it unique among zooxanthellae (LaJeunesse, 2005).
C3n-hh
- Reported Depth Range: 15m (49 feet)
- Reported Geographical Range: Lord Howe Island, Australia
- Host Species: Seriatopora hystrix
- Comments: Wicks et al., 2010b
C3n-t
- Reported Depth Range: 15m (49 feet)
- Reported Geographical Range: Lord Howe Island, Australia
- Host Species: Seriatopora hystrix
- Comments: Wicks et al., 2010b
C3o
- Reported Depth Range: <5 – >15 meters (<16 – >49 feet)
- Reported Geographical Range: Barbados
- Host Species: Montastrea cavernosa
- Comments: Present in coral before and after bleaching event caused by warm water. LaJeunesse et al., 2009.
C3p
- Reported Depth Range: <5 – >15 meters (<16 – >49 feet)
- Reported Geographical Range: Barbados
- Host Species: Montastrea cavernosa
- Comments: Present in coral 3 months into a bleaching event caused by warm water. LaJeunesse et al., 2009.
C3u
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Acropora millepora (green), Acropora sp., Euphyllia ancora, and Turbinaria sp.
- Comments: Aquarium corals. Smith et al., 2009
C3w
- Reported Depth Range: 2-3 meters (6-10 feet)
- Reported Geographical Range: Lord Howe Island, Australia
- Host Species: Acropora cuneata; Goniastrea favulus
- Comments: Wicks et al., 2010b
C3x
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Acropora sp., Acropora yongei
- Comments: Aquarium coral. Smith et al., 2009
C4
- Reported Depth Range: 0.3 – 25 meters (1 – 82 feet)
- Reported Geographical Range: Caribbean; Kenya
- Host Species: Acropora valida, Coscinarea mcneilli, Porites sp., Porites furcata, Strombus gigas (conch).
- References: LaJeunesse, 2004; Visram and Douglas, 2006
C5
- Reported Depth Range: 0.3 -15 meters (1 – 49 feet)
- Reported Geographical Range: Kenya; Caribbean
- Host Species: From a Kenyan (western Pacific) Acropora hyacinthus (Visram and Douglas, 2006) and a Caribbean Zoanthus sp. (LaJeunesse, 2005).
- Comments: Evidence suggests this clade is widely distributed.
C5a
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Agaricid corals
- Comments: A specialist zooxanthella (LaJeunesse, 2003)
C5b
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Agaricid corals
- Comments: A specialist zooxanthella (LaJeunesse, 2003)
C6
- Reported Depth Range: 0.3-10 meters (1 – 33 feet)
- Reported Geographical Range: Kenya; Caribbean
- Host Species: Acropora valida; Mycetophyllia
- Comments: C6 is apparently widely distributed (?), but only rarely reported. Visram and Douglas (2006) found this clade in a Kenyan Acropora valida, and LaJeunesse (2003) found it in a Caribbean stony coral Mycetophyllia.
C7
- Reported Depth Range: 0.3-25 meters (1 – 82 feet)
- Reported Geographical Range: Belize; U.S. Virgin Islands; Kenya, Africa
- Host Species: Montastrea sp., Montastrea annularis, Montastrea faveolata, Montastrea franksii, and Pocillopora damicornis
- Comments: Montastrea annularis (8-15m) and Montastraea faveolata (8-25m) in Belize (Warner, 2006) and in the stony coral Pocillopora damicornis (0.3-8m) in Kenya (Visram and Douglas, 2006).
C7a
- Reported Depth Range: >15 meters (>49 feet)
- Reported Geographical Range: Barbados, Caribbean
- Host Species: Montastrea annularis; Siderastrea siderea
- Comments: LaJeunesse et al., 2009
C8
- Reported Depth Range: 0.3 – 25 meters (1 – 82 feet)
- Reported Geographical Range: Kenya, Western Pacific, Great Barrier Reef
- Host Species: Pocillopora damicornis, Stylophora pistillata, Stylophora sp.
- Comments: Pocillopora damicornis (Kenya; 0.3-8m; Visram and Douglas, 2006), Western Pacific Stylophora sp. (5-25m; LaJeunesse et. al., 2003) and a GBR Stylophora pistillata (LaJeunesse et al., 2004). LaJeunesse believes C8 evolved from C1c (itself a variant of Clade C1).
- Stylophora pistillata colonies (at depths of 3 -18m; 10-59 feet) containing C8 bleached at 28.1°C (82.6°F), losing 60-70% of their zooxanthellae, although they remained apparently healthy. Sampayo et al. (2008), on studies conducted at Heron Island, southern Great Barrier Reef. More tolerant of warm water than clades C35, C35a, and C79.
C8a
- Reported Depth Range: 3 – 18 meters (10 – 59 feet)
- Reported Geographical Range: Southern Great Barrier Reef
- Host Species: Stylophora sp. and Stylophora pistillata
- Comments: LaJeunesse (2003; 2004) reports a Stylophora sp. and Stylophora pistillata to contain this clade. It seems restricted to deeper depths (~10m) and is thermally tolerant (Sampayo et al., 2008). A ‘sub-clade’ of C8 (LaJeunesse, 2005). See comments in ‘C8’ (above).
- Stylophora pistillata colonies (at depths of 3 -18m; 10-59 feet) containing C8a bleached at 28.1°C (82.6°F), losing 60-70% of their zooxanthellae, although they remained apparently healthy. Sampayo et al. (2008), on studies conducted at Heron Island, southern Great Barrier Reef. More tolerant of warm water than clades C35, C35a, and C79.
C8b
- Reported Depth Range: 2-3 meters (7 – 10 feet)
- Reported Geographical Range: Western Pacific
- Host Species: Stylophora sp.
- Comments: This zooxanthella strain is found in a stony coral Stylophora sp. but at shallower depths (2-3m) than Clade C8a (LaJeunesse et. al., 2003). A ‘sub-clade’ of C8 (LaJeunesse, 2005). See comments in ‘C8’ (above).
C8c
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Stylophora pistillata (green with brown polyps) and Stylophora pistillata (pink morph)
- Comments: Aquarium corals. Smith et al., 2009
C9
- Reported Depth Range: 0.3 – 8 meters (1 – 26 feet)
- Reported Geographical Range: Kenya; Caribbean
- Host Species: Porites divaricata, Pocillopora damicornis
- Comments: Pocillopora damicornis from Kenya (Visram and Douglas, 2006) and Porites divaricata (colored yellow or brown) from the Caribbean (LaJeunesse et. al., 2003).
C10
- Reported Depth Range: 0.3 – 15 meters (1 – 49 feet)
- Reported Geographical Range: Kenya; Caribbean
- Host Species: Pocillopora damicornis from Kenya (Visram and Douglas, 2006), Porites porites and a Porites sp. from the Caribbean (LaJeunesse et. al., 2003). Depths ranged from 0.3 to 15m.
C10a
- Reported Depth Range: 2-5 meters (7 – 16 feet)
- Reported Geographical Range: Caribbean
- Host Species: Porites sp.
- Comments: LaJeunesse et. al., 2003
C11
- Reported Depth Range: 0.3 -25 meters (1 – 82 feet)
- Reported Geographical Range: Caribbean; Bahamas, Kenya
- Host Species: Mussa sp., Mycetophyllia sp., Pocillopora damicornis, Scolymia sp., and Scolymia cubensis
- Comments: LaJeunesse et. al., 2003; Visram and Douglas, 2006
C12
- Reported Depth Range: 0.3 – >12 meters (1 – >40 feet)
- Reported Geographical Range: Kenya; Caribbean
- Host Species: Pocillopora damicornis from Kenyan waters at depths of 0.3 – 8m (Visram and Douglas 2006) as well as Caribbean Acropora cervicornis and Montastraea faveolata corals (at depths greater than 10m; LaJeunesse et. al., 2003)
- Comments: Evidence suggests this far-flung clade is pandemic.
C13
- Reported Depth Range: 0-5 meters (0 – 16 feet)
- Reported Geographical Range: Kenya; Caribbean
- Host Species: Pocillopora damicornis Kenya and a Porites sp. (Caribbean, 0.5-2m). References are Visram and Douglas, 2006 and LaJeunesse et. al., 2003, respectively.
- Comments: Evidence suggests this far-flung clade is pandemic.
C14
- Reported Depth Range: 0.5 – 2 meters (1.5-7 feet)
- Reported Geographical Range: Kenya; Caribbean
- Host Species: Pocillopora damicornis Kenya and a Porites sp. (Caribbean, 0.5-2m). References are Visram and Douglas, 2006 and LaJeunesse et. al., 2003, respectively.
- Comments: Evidence suggests this far-flung clade is pandemic.
C15
- Reported Depth Range: 1-396m (3-1,295 feet)
- Reported Geographical Range: Western Pacific, Indo-Pacific, Great Barrier Reef, Hawai’i
- Hosts: Aglaophenia (hydroid), Anacropora spinosa, Anphanipathes sp., Heteroxenia, Montipora sp., Montipora confusa, Montipora digitata, Montipora digitata (from Palau) Montipora digitata (green, from Solomon Islands), Montipora digitata (red, from Solomon Islands), Montipora capricornis, Montipora capricornis (brown, from Solomon Islands), Montipora sp. (green, plating), Montipora spongodes, Montipora spongodes (green), Montipora stellata, Myriopathes sp. (Wagner et al., 2010), Pocillopora damicornis, Porites sp., Porites brighami, Porites compressa, Porites cylindrica, Porites lutea (evermanni), and Porites lobata, Porites rus, and Seriatopora hystrix (brown with green polyps).
- C15 is also found in Aglaophenia, Heteroxenia (western Pacific, both sampled at 1.0 -15.m LaJeunesse et al., 2003), Montipora (GBR, Pochon, 2004), Montipora (probably M. digitata at 1.0-15.0, LaJeunesse et al., 2003 ), Pocillopora damicornis (0.3-8.0m, Kenya, Visram and Douglas, 2006), Porites sp. from various Pacific locations (LaJeunesse et. al., 2003 and Pochon et al., 2004), Porites brighami (at 20m in Hawaii, LaJeunesse et al., 2003); Porites compressa (Hawaii, 15-25m, LaJeunesse et al., 2003), Porites cylindrica (GBR, LaJeunesse et al., 2003), Porites evermanni (now P. lutea, Hawaii, 5.0-20.0m, LaJeunesse, 2004), Porites lobata (Hawaii, 2-20m, LaJeunesse, 2003), Porites lutea (purple variant, 1.5m, Hawaii, Smith et al., 2009).
- Comments: C15 is considered tolerant of high and low temperatures. It is also tolerant of extremes in light intensity – corals containing C15 can be found in the most shallow of tidepools as well as hosts (black corals) in extremely deep water (~1,300 feet). Often found in Pacific Porites spp., though not exclusive of other genera. See Figures 6 and 7 (above) and 8 (below) for further information. Possibly a variant of Clade C3 (LaJeunesse, 2005).
PAM fluorometry work found onset of photosaturation ranging from 250-400 µmole photons·m²·sec (~12,500 – 20,000 lux) and onset of photoinhibition ranging from ~350 to ~750 µmole photons·m²·sec (~17,500 – 37,500 lux) in a Hawaiian shallow-water (1 meter depth) Porites lobata and Porites lutea specimens, respectively (ITS 2 analysis on P. lutea by Smith et al., 2009; PAM fluorometry by Riddle, 2007: http://www.advancedaquarist.com/2007/3/aafeature1/
As a footnote, a Hawaiian Porites compressa specimen was transplanted from depth to about 1m – it retained its C15 zooxanthellae.
C15a
- Reported Depth Range: 10 meters (33 feet)
- Reported Geographical Range: Eastern Pacific (Panama)
- Hosts: Porites
- Comments: A variant of C15. LaJeunesse et al., 2003.
C15b
- Reported Depth Range: 1-25 meters (3 – 82 feet), but see note below
- Reported Geographical Range: Hawai’i
- Hosts: Porites sp., Porites compressa, Porites rus, ‘encrusting octocoral’, and the soft coral Sarcothelia edmonsoni
C15c
- Reported Depth Range: 10-15 meters (33-49 feet)
- Reported Geographical Range: Hawai’i
- Hosts: Porites sp., Porites rus
- Comments: A variant of C15.
C15d
- Reported Depth Range: >10 meters (>33 feet)
- Reported Geographical Range: Central Pacific
- Hosts: Porites sp.
C15e
- Reported Depth Range: 10 – 17 meters (33 – 49 feet)
- Reported Geographical Range: Western Caribbean, Great Barrier Reef
- Hosts: Heteroxenia sp., Millepora sp., Millepora tenella
- Comments: A variant of C15 (LaJeunesse, 2004).
C15f
- Reported Depth Range: 1 – 8 meters (3 – 26 feet)
- Reported Geographical Range: Western Pacific, Great Barrier Reef
- Hosts: Montipora sp., Montipora monasteriata
- Comments: A variant of C15 (LaJeunesse, 2004).
C15g
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Montipora digitata
- Comments: Aquarium coral. Smith et al., 2009
C15h
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Montipora sp., Montipora sp. (green/brown, plating), Montipora sp. (“Superman”), Montipora capricornis, Montipora capricornis (purple, plating), Montipora capricornis (purple/brown), and Montipora capricornis (orange),
- Comments: Mostly aquarium corals. Smith et al., 2009
C15m
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Montipora digitata (bright orange)
- Comments: Aquarium coral. Smith et al., 2009
C16
- Reported Depth Range: 0.3-25 meters (1-82 feet)
- Reported Geographical Range: Kenya; Belize; Western Caribbean
- Hosts: Pocillopora damicornis, Porites sp., Siderastrea intersepta, Stephanocoenia sp.
- Comments: Siderastrea intersepta (Warner 2006) and Stephanocoenia sp. (LaJeunesse et. al., 2003). Both from the western Caribbean.
C16a
- Reported Depth Range: 12-18 meters (39 – 59 feet)
- Reported Geographical Range: Central Caribbean
- Hosts: Stephanocoenia sp.
- Comments: LaJeunesse et. al., 2003
C17
- Reported Depth Range: 3-20 meters (10 – 65 feet)
- Reported Geographical Range: Eastern Pacific; Great Barrier Reef
- Hosts: Montipora sp., Montipora aequituberculata, Montipora capitata, Montipora foliosa, Montipora monasteriata, Porites sp.
- Note: Zooxanthellae were identified as C17 in brown and purple Montipora monasteriata specimens.
C18
- Reported Depth Range: ?
- Reported Geographical Range: Probably Pacific
- Hosts: ?
- Comments: Listed by LaJeunesse (2005) without specifics.
C19
- Reported Depth Range: 1 meter (3 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Hosts: Acropora sp., Acropora florida
C20
- Reported Depth Range: 4 meters (13 feet)
- Reported Geographical Range: Western Pacific
- Host:Zoanthus sp.
C21
- Reported Depth Range: 2-10 meters (7 – 33 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Hosts: Acropora latistella, Archelia sp., Cyphastrea sp., Echinophyllia sp., Echinopora sp., Echinopora lamellosa, Favia sp., Favites sp., Fungia sp., Leptoseris explanata, Merulina sp., Montipora sp., Montipora faveolata, Montipora flabellata, Montipora monasteriata, Pachyseris speciosa, and Pavona explanata.
- Note: The M. monasteriata examined was living in a cave, and was red-brown in color. High light brown and purple M. monasteriata specimens contain Clade C17 zooxanthellae (considered to be a generalist zooxanthella).
- Also found in many aquarium corals: Acropora sp., Acropora sp. (aquamarine blue), Acropora sp. (green morph), Acropora granulosa (purple/green; found in conjunction with Clade C94a), Acropora microphthalma, Acropora millepora, Acropora tenuis, Montipora capricornis, and Pachyseris gemmae.
C21a
- Reported Depth Range: 1 – 20 meters (3 – 65 feet)
- Reported Geographical Range: Hawai’i, Okinawa, Western Pacific
- Hosts: Corals Cyphastrea, Echinophyllia, Favites, Galaxea, Hydnophora, Lobophyllia, Lobophyllia corymbosa, Pectinia, Symphyllia (all LaJeunesse, et al., 2003), and the stony coral Turbinaria (LaJeunesse, 2004) have been found to contain C21a. Predominantly, if not exclusively, found in the northern hemisphere and often in waters around Okinawa, Japan.
C21-b1
- Reported Depth Range: 11-396 meters (36 – ~1,300 feet)
- Reported Geographical Range: Hawai’i
- Hosts: Acanthopathes undulata, Anphanipathes sp., Antipathes griggi, Antipathes grandis, Bathypathes sp., Cirrhipathes anguina, Myriopathes sp., Stichopathes sp.
- Comments: Very closely related to Clade 21. Wagner et al., 2010
C22
- Reported Depth Range: 3 -10 meters (10 – 33 feet)
- Reported Geographical Range: Great Barrier Reef
- Hosts: Found (so far) in Lobophyllia, Lobophyllia corymbosa and Turbinaria sp., Turbinaria heronensis, and Turbinaria peltata.
- Comments: LaJeunesse et al., 2003 & 2004
C22a
- Reported Depth Range: 5 – 15 meters (16 – 49 feet)
- Reported Geographical Range: Kermadec Island, New Zealand; southern Great Barrier Reef
- Hosts: Turbinaria frondens
- Comments: Results of testing of ITS1 and ITS2 regions of DNA will both identify the clade as C1c. Wicks et al., 2010b
C23
- Reported Depth Range: 4 meters (13 feet)
- Reported Geographical Range: Pacific
- Hosts: Found in octocorals Briareum and Isis spp. (LaJeunesse, 2005).
- Comments: Believed to have evolved from Clade C1.
C24
- Reported Depth Range: 3 meters (10 feet)
- Reported Geographical Range: Red Sea
- Hosts: Isolated from the zoanthid Palythoa.
- Comments: Believed to have evolved from Clade C1, and co-evolved along with Clade 41 (found in a number of Red Sea stony corals), LaJeunesse, 2005.
C25
- Reported Depth Range: 3 meters (10 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Hosts: Heteractis, Heteractis magnifica anemone.
- Comments: A rare or host-specific clade, believed to have evolved from Clade C1, and co-evolved along with C59 (found in some Seriatopora specimens, LaJeunesse, 2005).
C26
- Reported Depth Range: 3-20 meters (10 – 65 feet)
- Geographical Range: Great Barrier Reef and Hawai’i
- Host Species: Reportedly found only in Montipora species, including M. capitata and M. stellata.
- References: LaJeunesse 2003; 2004.
C26a
- Reported Depth Range: >15 meters (> 49 feet)
- Reported Geographical Range: Great Barrier Reef, Western Pacific, Hawai’i, Central Pacific
- Hosts: Isolated from Montipora species, Montipora capitata (collected in waters deeper than 15m), Montipora hispida, Montipora monasteriata, and Montipora turtlensis.
- Also found in aquarium corals: Montipora sp., Montipora aequituberculata, and Montipora capricornis.
- Comments: LaJeunesse (2004) believed to have evolved from C21.
C26b1
- Reported Depth Range: 11-396 meters (36 – ~1,300 feet)
- Reported Geographical Range: Hawai’i
- Hosts: Acanthopathes undulata, Anphanipathes sp., Antipathes griggi, Antipathes grandis, Bathypathes sp., Cirrhipathes anguina, Myriopathes sp., Stichopathes sp.
- Comments: Very closely related to Clade 26. Wagner et al., 2010
C26b2
- Reported Depth Range: 11-396 meters (36 – ~1,300 feet)
- Reported Geographical Range: Hawai’i
- Hosts: Acanthopathes undulata, Anphanipathes sp., Antipathes griggi, Antipathes grandis, Bathypathes sp., Cirrhipathes anguina, Myriopathes sp., Stichopathes sp.
- Reference: Wagner et al., 2010
C26b3
- Reported Depth Range: 11-396 meters (36 – ~1,300 feet)
- Reported Geographical Range: Hawai’i
- Hosts: Acanthopathes undulata, Anphanipathes sp., Antipathes grandis, Bathypathes sp., Cirrhipathes anguina
- Reference: Wagner et al., 2010
C27
- Reported Depth Range: 1 – 25 meters (3 – feet)
- Reported Geographical Range: Pacific and Central Pacific (Hawaii)
- Hosts: Alveopora sp., Coscinaraea sp., Fungia sp., Fungia danai, Hydnophora sp., Hydnophora exesa, hydrocoral Millepora sp., Pachyseris sp., Pachyseris speciosa, Pavona, Pavona duerdeni, and Pavona varians. Also found in aquarium corals: Euphyllia divisa, and Fungia sp.
- Comments: Believed to have evolved from C21. PAM fluorometry work found onset of photosaturation at 110 µmole photons·m²·sec (~5,500 lux) and onset of photoinhibition at ~350 µmole photons·m²·sec (~17,500 lux) in a Hawaiian Pavona varians specimen (ITS 2 analysis by Smith et al., 2009; PAM fluorometry by Riddle, 2007): http://www.advancedaquarist.com/2007/3/aafeature1/
C28
- Reported Depth Range: 3 meters (10 feet)
- Reported Geographical Range: Great Barrier Reef
- Hosts: Porites sp. and Porites annae (LaJeunesse et al., 2003; 2004, respectively)
C29
- Reported Depth Range: 1 meter (3 feet)
- Reported Geographical Range: Central and eastern Pacific
- Hosts: Zoanthus sp. (1m) and Zoanthus pacificus (Hawaii, 1 m)
- References: LaJeunesse et al., 2003 and LaJeunesse et al., 2004.
C30
- Reported Depth Range: 1-10 meters (~3 – 33 feet)
- Reported Geographical Range: Okinawa, Japan; Western Pacific
- Host Species: Montipora sp., Montipora efflorescens
C31
- Reported Depth Range: 1-20 meters (~3 – 65 feet; ~1,300 feet for a black coral Stichopathes species)
- Reported Geographical Range: Pandemic: Caribbean (Belize); Hawai’i, Okinawa, Japan; Great Barrier Reef
- Host Species: Montastrea annularis, Montipora sp., Montipora capitata, Montipora danae, Montipora patula, Montipora turtlensis, Montipora venosa, Montipora verrucosa, and deep-water Stichopathes sp.. It is possible this clade becomes parasitic when in low light conditions.
C31a
- Reported Depth Range: 1-10 meters (~3 – 33 feet)
- Reported Geographical Range: Western Pacific; Okinawa, Japan.
- Host Species: Montipora sp., Montipora venosa
- Comments: LaJeunesse et al., 2003; 2004
C31b
- Reported Depth Range: 10-17 meters (33 – 56 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Host Species: Alveopora sp.; Alveopora fenestrata
- Comments: LaJeunesse et al., 2003; 2004
C31c
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Montipora capitata
- Comments: Aquarium coral. Smith et al., 2009
C32
- Reported Depth Range: 1-3 meters (~3 – 10 feet)
- Reported Geographical Range: Central Pacific (Hawai’i)
- Host Species: Montipora sp.; Montipora flabellata
- Comments: LaJeunesse et al., 2003; 2004
C32a
- Reported Depth Range: 10-20 meters (33 – 65 feet)
- Reported Geographical Range: Hawai’i
- Host Species: Montipora flabellata
- Comments: M. flabellata often lives in waters shallower than collection depths listed above.
C33
- Reported Depth Range: 12-15 meters (39 – 49 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Host Species: Pocillopora sp.
- Comments: LaJeunesse et al., 2003
C34
- Reported Depth Range: 18-25 meters (59 – 82 feet)
- Reported Geographical Range: Central Pacific; Hawai’i
- Host Species: Pocillopora sp., Pocillopora molokensis
- Comments: Pocillopora molokensis is restricted to deeper waters, suggesting Clade 34 prefers less light, UVR, or lower temperatures.
C35
- Reported Depth Range: 12-15 meters (39 – 114 feet)
- Reported Geographical Range: Western Pacific, Great Barrier Reef
- Host Species: Seriatopora sp.; Stylophora pistillata
- Comments: Sensitive to heat stress. Stylophora pistillata colonies (at depths of 3 -6m; 10-20 feet) containing C35 suffered significant bleaching at 28.1°C (82.6°F), losing ~100% of their zooxanthellae. Studies conducted at Heron Island, southern Great Barrier Reef. Less tolerant of warm water than clades C8, C8a, and C78 (also found there in S. pistillata colonies; Sampayo et al., 2008).
C35a
- Reported Depth Range: 12-15 meters (39 – 49 feet)
- Reported Geographical Range: Great Barrier Reef
- Host Species: Stylophora pistillata
- Comments: Sensitive to heat stress. Stylophora pistillata colonies (at depths of 3 -6m; 10-20 feet) containing C35 suffered significant bleaching at 28.1°C (82.6°F), losing ~100% of their zooxanthellae. Studies conducted at Heron Island, southern Great Barrier Reef. Less tolerant of warm water than clades C8, C8a, and C78 (also found there in S. pistillata colonies; Sampayo et al., 2008).
C36
- Reported Depth Range: 2 meters (7 feet)
- Reported Geographical Range: West Indies; Pacific
- Host Species: Coeloseris sp., Goniastrea sp.
C37
- Reported Depth Range: 2 meters (7 feet)
- Reported Geographical Range: Western Pacific
- Host Species: Stylophora sp.
C38
- Reported Depth Range: >10m (>33 feet)
- Reported Geographical Range: Eastern Caribbean
- Host Species: Acropora sp.
C38a
- Reported Depth Range: >10 meters (>33 feet)
- Reported Geographical Range: Eastern Caribbean
- Host Species: Acropora sp.
C39
- Reported Depth Range: 6-7 meters (20 – 23 feet)
- Reported Geographical Range: Red Sea
- Host Species: Diploastrea sp.; ‘Mussidae’
C40
- Reported Depth Range: 1-17 meters (~3 – 56 feet)
- Reported Geographical Range: Great Barrier Reef
- Host Species: Echinopora lamellosa, Echinopora hirsutissima, Mycedium elephantotus, Symphyllia radians, Symphyllia recta, Turbinaria frondens, Turbinaria reniformis, and Turbinaria stellulata.
- Also reported from aquarium corals: Acropora sp., Pectinia sp. (Smith et al., 2009)
C40a
- Reported Depth Range: 10-17 meters (33 – 56 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Host Species: Echinopora sp.; Echinopora horrida
C40b
- Reported Depth Range: 1-6 meters (~3 – 20 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Host Species: Echinopora sp.; Echinopora lamellosa
C41
- Reported Depth Range: 2-8 meters (7 – 26 feet)
- Reported Geographical Range: Red Sea, West Indies
- Host Species: Acropora sp., Acropora hemprichii, Platygyra sp., Plesiastrea sp., Plesiastrea laxa, Turbinaria sp.
C42
- Reported Depth Range: 0-4 meters (0 – 49 feet)
- Reported Geographical Range: Pacific; Great Barrier Reef
- Host Species: Pocillopora damicornis
C42a
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: ?
- Comments: Listed by LaJeunesse (2005) without further comment.
C42b
- Reported Depth Range: 0.5 meter (~1.5 feet)
- Reported Geographical Range: Western Pacific
- Host Species: Pocillopora sp.
C43
- Reported Depth Range: 15 meters (49 feet)
- Reported Geographical Range: Eastern Caribbean
- Host Species: Porites sp.
C44
- Reported Depth Range: 4-15 meters (13 – 49 feet)
- Reported Geographical Range: Northern Caribbean
- Host Species: Porites divaricata
C44a
- Reported Depth Range: 4-15 meters (13 – 49 feet)
- Reported Geographical Range: Northern Caribbean
- Host Species: Porites divaricata
C45
- Reported Depth Range: 15 meters (49 feet)
- Reported Geographical Range: Northern Caribbean
- Host Species: Porites sp.
C45a
- Reported Depth Range: 4-5 meters (13 – 16 feet)
- Reported Geographical Range: Central Caribbean
- Host Species: Porites porites
C46
- Reported Depth Range: 4-5 meters (13 – 16 feet)
- Reported Geographical Range: Central Caribbean, US Virgin Islands
- Host Species: Siderastrea sp., Siderastrea radians
C46a
- Reported Depth Range: 4-5 meters (13 – 16 feet)
- Reported Geographical Range: Caribbean
- Host Species: Siderastrea sp.
C47
- Reported Depth Range: 25 meters (82 feet)
- Reported Geographical Range: Western Caribbean
- Host Species: Porites porites
C48
- Reported Depth Range: 17 meters (56 feet)
- Reported Geographical Range: Eastern Caribbean
- Host Species: Mycetophyllia sp.
C49
- Reported Depth Range: 15 meters (49 feet)
- Reported Geographical Range: Northern Caribbean
- Host Species: Mycetophyllia sp.
C50
- Reported Depth Range: 15 meters (49 feet)
- Reported Geographical Range: Northern Caribbean
- Host Species: Diploria sp.
C51
- Reported Depth Range: 2 meters (7 feet)
- Reported Geographical Range: Northern Caribbean
- Host Species: Mycetophyllia sp.
C52
- Reported Depth Range: 25 meters (82 feet)
- Reported Geographical Range: Western Caribbean
- Host Species: Agaricia agaricities
C53
- Reported Depth Range: 15 meters (49 feet)
- Reported Geographical Range: Western Caribbean
- Host Species: Manicina sp.
C54
- Reported Depth Range: 8 meters (26 feet)
- Reported Geographical Range: Western Caribbean (Belize)
- Host Species: Siderastrea intersepta
C54a
- Reported Depth Range: 12-18 meters (39-59 feet)
- Reported Geographical Range: Central Caribbean
- Host Species: Favia fragum
C55
- Reported Depth Range: 1-10 meters (~3-33 feet)
- Reported Geographical Range: Western Pacific; Okinawa, Japan
- Host Species: Platygyra sp.; Porites sp.
C56
- Reported Depth Range: 1-10 meters (~3-33 feet)
- Reported Geographical Range: Western Pacific; Okinawa, Japan
- Host Species: Porites sp.; Porites lichen
C56a
- Reported Depth Range: 1-10 meters (~3-33 feet)
- Reported Geographical Range: Western Pacific; Okinawa, Japan
- Host Species: Porites cylindrica
C57
- Reported Depth Range: 3-10 meters (~10-33 feet)
- Reported Geographical Range: Western Pacific; Okinawa, Japan
- Host Species: Millepora sp.
C58
- Reported Depth Range: 1-10 meters (~3-33 feet)
- Reported Geographical Range: Western Pacific; Okinawa, Japan
- Host Species: Montipora sp.; Montipora mollis
C59
- Reported Depth Range: 3 meters (10 feet)
- Reported Geographical Range: Western Pacific; Okinawa, Japan
- Host Species: Seriatopora sp., Seriatopora hystrix
C60
- Reported Depth Range: 1-8 meters (~3-26 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef; Lord Howe Island
- Host Species: Porites sp.; Porites annae; Porites cylindrica; Porites heronensis, Porites vaughani
C61
- Reported Depth Range: 10-17 meters (~33-56 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Host Species: Montipora sp.; Montipora grisea
C62
- Reported Depth Range: 1-17 meters (~3-56 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Host Species: Zoanthus sp.
C64
- Reported Depth Range: 1 – 17 meters (~3 feet – 56 feet)
- Reported Geographical Range: Great Barrier Reef
- Host Species: Anthelia, Heteroxenia, Klyxum, and Xenia spp.
C65
- Reported Depth Range: 3 – 17 meters (10 – 56 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Host Species: Hicksonella sp., Hicksonella expansa, Lobophyllia, Sarcophyton, Sinularia spp., and from a Sinularia (with green branch tips) maintained in an aquarium.
C65a
- Reported Depth Range: 3 – 15 meters (10 – 49 feet)
- Reported Geographical Range: Western Pacific
- Host Species: Hicksonella, Lobophyllia, Sarcophyton, Sinularia spp
C66
- Reported Depth Range: 1 -2 meters (3 -7 feet)
- Reported Geographical Range: Red Sea, Eastern Pacific, Great Barrier Reef
- Host Species: Porites panamensis, Turbinaria sp., ‘Xeniidae’
- Reference: LaJeunesse et al., 2003; Barneah, 2007
C66a
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: ?
- LaJeunesse (2005) refers to this clade without specifics.
C66b
- Reported Depth Range: 0.5-1 meters (1.6 – 3 feet)
- Reported Geographical Range: Eastern Pacific
- Host Species: Porites panamensis
C66c
- Reported Depth Range: 4-10 meters (13 – 33 feet)
- Reported Geographical Range: Red Sea
- Host Species: ‘Flatworm’
C67
- Reported Depth Range: 1-8 meters (~3 -26 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Host Species: Heteractis magnifica (anemone)
C68
- Reported Depth Range: 1-6 meters (~3 -20 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Host Species: Heteractis magnifica (anemone)
C69
- Reported Depth Range: 6 meters (20 feet)
- Reported Geographical Range: Western Pacific
- Host Species: Stichodactyla (anemone)
C69a
- Reported Depth Range: 1-8 meters (~3 – 26 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Host Species: Anemones Stichodactyla sp. and Stichodactyla gigantea
C70
- Reported Depth Range: 1-10 meters (3 – 33 feet)
- Reported Geographical Range: Western Pacific; Okinawa, Japan
- Host Species: Unknown anemone
C71
- Reported Depth Range: 3 meters (10 feet)
- Reported Geographical Range: Western Pacific
- Host Species: Sinularia sp.
C71a
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: ?
- Comment: Listed by LaJeunesse 2005 without elaboration.
C72
- Reported Depth Range: 20 meters (65 feet)
- Reported Geographical Range: Red Sea
- Host Species: Stylophora sp.; Stylophora pistillata
C73
- Reported Depth Range: 0.5 meters (~0.5 feet)
- Reported Geographical Range: Western Pacific
- Host Species: Montipora digitata
C74
- Reported Depth Range: 1-6 meters (~3 – 20 feet)
- Reported Geographical Range: Red Sea
- Host Species: Acropora hemprichi, an unidentified ‘flatworm’ and the stony coral Plesiastrea laxa
C75
- Reported Depth Range: 5-14 meters (16 – 46 feet)
- Reported Geographical Range: Eastern Pacific
- Host Species: Porites panamensis
C76
- Reported Depth Range: 4-5 meters (13 – 16 feet)
- Reported Geographical Range: Central Caribbean
- Host Species: Isophyllia sinuosa
C77
- Reported Depth Range: 4-5 meters (13 – 16 feet)
- Reported Geographical Range: Central Caribbean
- Host Species: Isophyllastrea rigida
C78
- Reported Depth Range: 0.5-4 meters (0.5 – 13 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Host Species: Stylophora sp. and Stylophora pistillata
- Comments: Thermally tolerant, relative to Clades C79 and C35a (Sampayo et al., 2008).
C78a
- Reported Depth Range: 0.5-4 meters (0.5 – 13 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Host Species: Stylophora sp. and Stylophora pistillata
- Comments: Stylophora pistillata colonies (at depths of 3 -18m; 10-59 feet) containing C8a bleached at 28.1°C (82.6°F), losing 60-70% of their zooxanthellae, although they remained apparently healthy. Sampayo et al. (2008), on studies conducted at Heron Island, southern Great Barrier Reef. More tolerant of warm water than clades C35, C35a, and C79. Incorrectly identified as C1 in a previous work (Sampayo et al., 2007).
C79
- Reported Depth Range: 12-18 meters (40 – 59 feet)
- Reported Geographical Range: Western Pacific; Great Barrier Reef
- Host Species: Stylophora sp.; Stylophora pistillata
- Comments: Sensitive to heat stress. Stylophora pistillata colonies (at depths of 15-18m; 10-20 feet) containing C35 suffered significant bleaching at 28.1°C (82.6°F), losing ~100% of their zooxanthellae. Studies conducted at Heron Island, southern Great Barrier Reef. Less tolerant of warm water than clades C8, C8a, and C78 (also found there in S. pistillata colonies; Sampayo et al., 2008).
C84
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: ?
- Listed by LaJeunesse 2005 without elaboration.
C84a
- Reported Depth Range: 2-6 meters (~7 – 20 feet)
- Reported Geographical Range: Central Great Barrier Reef
- Host Species: Anthelia sp.
C88a
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Montipora sp., and Montipora capricornis (green morph).
- Comments: Aquarium coral. Smith et al., 2009.
C89
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Echinopora sp. and Polyphyllia sp.
- Comments: Aquarium coral. Smith et al., 2009
C94a
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Acropora sp., Acropora sp. (blue), Acropora sp. (blue branch tips), Acropora sp. (green tabletop), Acropora cervicornis (?), Acropora granulosa (along with Clade C21), Acropora humilis, Acropora millepora (pink), and Acropora valida (purple branch tips), as well as a flat worm taken one of the specimens listed above (an interesting observation – is the flat worm parasitic, or simply eats ejected zooxanthellae?).
- Comments: Aquarium corals. Smith et al., 2009
C96
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Montipora sp.
- Comments: Aquarium coral. Smith et al., 2009
C100
- Reported Depth Range: 2-3m (10-20 feet)
- Reported Geographical Range: Lowe Howe Island, south GBR
- Host Species: Acropora sp., Acropora cuneata, and Pocillopora damicornis
- Comments: Pocillopora damicornis colonies harboring C100 were tolerant of temperatures between 15°C and 29°C (~59 and 84°F) and bleached outside this range. PAM fluorometry revealed C100 is more sensitive to warmer temperatures (especially in the presence of light of up to 2,000 µmol·m²·sec). Wicks et al., 2010. Results of testing of ITS1 and ITS2 regions of DNA will both identify the clade as C100.
C101
- Reported Depth Range: ?
- Reported Geographical Range: ?
- Host Species: Acropora formosa (now A. muricata; blue morph), Acropora valida (purple morph), Acropora sp. (“Miami Orchid”), and bright-yellow Acropora sp.
- Comments: Aquarium corals. Smith et al., 2009
C102
- Reported Depth Range: 2-3 meters (10-20 feet)
- Reported Geographical Range: Lord Howe Island, south GBR
- Host Species: Acropora species, Acropora cuneata
- Comments: Wicks et al., 2010a; Wicks et al., 2010b
C103
- Reported Depth Range: 1-3 meters (3-10 feet)
- Reported Geographical Range: Lord Howe Island, south GBR
- Host Species: Pocillopora damicornis
- Comments: C103 has, so far, been reported in only Pocillopora damicornis colonies, and even then those in ‘deeper’, more turbid waters. Wicks et al., 2010a. Results of testing of ITS1 and ITS2 regions of DNA will both identify the clade as C103.
C104
- Reported Depth Range: ?
- Reported Geographical Range: Lord Howe Island, south GBR
- Host Species: Cyphastrea serailia
- Comments: Wicks et al., 2010
C108
- Reported Depth Range: 2-3 meters (10-20 feet)
- Reported Geographical Range: Lord Howe Island, south GBR
- Host Species: Pocillopora damicornis
- Comments: Wicks et al., 2010
C111
- Reported Depth Range: 5 meters (16 feet)
- Reported Geographical Range: Northern Great Barrier Reef
- Host Species: Porites heronensis
- Comments: Wicks et al., 2010b. Results of testing of ITS1 and ITS2 regions of DNA will both identify the clade as C111.
C117
- Reported Depth Range: 5 meters (16 feet)
- Reported Geographical Range: Lord Howe Island, Australia
- Host Species: Porites heronensis
- Comments: Results of testing of ITS1 and ITS2 regions of DNA will both identify the clade as C117. Wicks et al., 2010b
C118
- Reported Depth Range: 2-3 meters (10-20 feet)
- Reported Geographical Range: Lord Howe Island, south GBR
- Host Species: Pocillopora damicornis (but see comments)
- Comments: Wicks et al., 2010a & b.
Clade D
Phylotype “D” are relatively resistant (generally) to bleaching (in comparison to many Clade C phylotypes), and, in fact, often found in areas that have suffered recent, severe bleaching episodes and hot environments.
Chen et al., 2003, found this clade within high latitude corals Oulastrea crispata and Goniastrea aspera inhabiting marginal sites (extreme temperatures, turbidity and irradiance); Baum et al. (2010) found Clade D (along with Clade A) in Florida Acropora cervicornis specimens inhabiting areas with wide temperature fluctuations, high nutrient loading and high sedimentation. This zooxanthellae is thus considered extremely stress tolerant and opportunistic (‘opportunisitic zooxanthella’ would be defined as one possessing traits or characteristics making it competitive in a marginal environment – such as high or low temperature, etc.).
On the other hand, Abrego et al. (2008) found a Clade D in Acropora tenuis to be less resistant to environmental extremes than C1 in other A. tenuis specimens. Is Clade C1 simply more robust and less prone to bleaching induced by relatively warm water temperature?
Is Clade D as described by Abrego et al. in reality a subclade in the D-class? These researchers used ITS1 portions to identify the clades, instead of the ITS2 portion of the RNA used by many other scientists.
Abrego et al. (2008) found a clade identified only as “D” to produce protective xanthophyll pigments.
Clade D is the proper classification for symbionts listed in earlier works by Carlos et al. (1999) and Toller (2001 a, b).
D
- Reported Depth Range: 2-23m (~7-75 feet)
- Reported Geographical Range: Caribbean (Panama); Eastern Pacific (Panama); Great Barrier Reef; Malaysia; Guam, American Samoa
- Hosts: Acropora millepora, Agaricia humilis, Diploria labyrinthiformis, Montastrea franksi, Montastrea faveolata, Pocillopora elegans, Seriatopora hystrix, Siderastrea sidereal Stephanocoenia michellini, and Stephanocoenia intersepta.
D1: Symbiodinium glynni
- Reported Depth Range: 0-3m (0-10 feet)
- Reported Geographical Range: Taiwan; Gulf of California; Eastern Pacific
- Hosts: Acropora palifera, Oulastrea crispata (Taiwan); Pocillopora spp. (Gulf of California). Also found in these corals in association with Clade C1 at depths of 8-15m (26-49 feet): Acropora palifera, Euphyllia ancora, Euphyllia parancora, Turbinaria mesenteria.Found in an Acropora sp. (orange morph) maintained in an aquarium.
D1a: Symbiodinium trenchi
- Reported Depth Range: 1-15m (3-49 feet) but up to 24 meters (79 feet) for the wire coral Antipathes griggi
- Reported Geographical Range: Pacific, Hawai’i, Western Caribbean (Belize); Eastern Caribbean (Barbados); southern Great Barrier Reef
- Hosts: Agaricia sp., Antipathes griggi, Diploria spp., Echinopora hirsutissima, Goniastrea favulus, Hydnophora microconus, Meandrina meandrites, Montastrea annularis, Montastrea cavernosa, Montastrea faveolata Montipora capitata (orange), Montipora patula, Nephthea, Porites astreoides, Porites divaricate, Porites porites, andSiderastrea siderea. Found in captive corals Euphyllia sp. and Hydnophora sp. (Smith et al., 2009).
- Comments: Thermally-tolerant, adaptable to low light, and opportunistic.
D1a-f
- Reported Depth Range: ?
- Reported Geographical Range: Pacific
- Hosts: Pocillopora damicornis and Seriatopora hystrix.
- Comments: Aquarium corals. (Smith et al., 2009).
D2
- Reported Depth Range: 0-8m (0-26 feet)
- Reported Geographical Range: Guam, Okinawa, Taiwan, Mexican Pacific, Kenya; northern Great Barrier Reef
- Hosts: Acropora sp., Acropora bruegmanni, Acropora cuneata, Acropora palifera, Galaxea fasicularis, Goniopora fruticosa, Pavona desucata, Pocillopora elegans, Pocillopora verrucosa.
- Comments: Clade D2 apparently prefers environments with high light.
Clade D206 (equivalent to D1a)
- Reported Depth Range: ?
- Reported Geographical Range: Caribbean
- Reported Hosts: Caribbean stony coral Porites divaricata
- Comment: The numerical portion of the clade ID is based on the length (base pairs or bp) of a variable region in the chloroplast 23 s rDNA genes, and not the ITS1 or ITS2 regions used by many researchers.
- Reference: Coffroth et al., 2010.
Clade E
Those zooxanthellae listed as Clade E in Toller et al., (2001) have been reclassified as Clade D. Symbiodinium muscatinei and S. californium (from the anemone Anthopleura) are sometimes listed as belonging to Clade E; they are listed as Clade B (above). Zwada and Jaffe (2003) found Caribbean Montastrea faveolata specimens (collected of depths of 7 to 11 meters) contained zooxanthellae belonging to Clade E.
Clade F
Normally found in foraminiferans, researchers were surprised when Clade Fr2 was found in isolated ‘daisy coral’ specimens (Alveopora japonica) in Korea. (Rodriguez-Lanetty et al., 2000). Another clade, F5, occurs in Montipora capitata, as well as Sinularia sp. (Guam), Meandrina meandrites (Jamaica) and a Floridian Porites astreoides (Rodriguez-Lanetty, 2003.
Reported Species in Clade F
Symbiodinium kawagutii. This zooxanthella species (designated as Clade F5) is found within the coral Montipora capitata (formerly M. verrucosa and found in Hawaii and Australia). No protective xanthophylls are produced as a response to super-saturating irradiance (Iglesias-Prieto and Trench, 1997), and this zooxanthella (and host) does poorly in high light intensity. It is interesting that both corals containing Clade F are found at higher latitudes.
F
- Reported Depth Range: ?
- Reported Geographical Range: Korea
- Hosts: Alveopora japonica
F5
- Reported Depth Range: ?
- Reported Geographical Range: Hawai’i; Great Barrier Reef
- Hosts: Montipora capitata (verrucosa)
- Reference: Rodriguez-Lanetty, 2003
- Comment: F5 is not tolerant of high light intensity, but there are reports of M. capitata specimens containing MAAs thus making them relatively tolerant of ultraviolet radiation. It is not known if these are obtained through diet or translocated from zooxanthellae.Pulse Amplitude Modulation fluorometry of a shallow-water Montipora capitata found a light saturation point of 110 µmol·m²·sec (Riddle, 2007: http://www.advancedaquarist.com/2007/3/aafeature1/
Fr2
- Reported Depth Range: ?
- Reported Geographical Range: Korea
- Host: Alveopora japonica
- Comments: Rodriguez-Lanetty, 2003
Clade G
Clade G has recently been found in soft corals (van Oppen, 2005a) and ‘giant’ sea anemones (LaJeunesse, in Pochon, 2005).
- Reported Depth Range: <18 meters (< 59 feet)
- Reported Geographical Range: Great Barrier Reef
- Hosts: ‘Giant’ sea anemones; Junceella fragilis, Euplexaura nuttingi, Stereonephthya sp. #1.
- Note: Stereonephthya sp. are generally considered to be azooxanthellate (that is, free of infection by Symbiodinium), however multiple sources list soft coral to contain zooxanthellae (if only rarely). Van Oppen et al., 2005a report the infected Stereonephthya to be bland in appearance and lacking the normal bright coloration.
In Closing
If you’re reading this, you’re one dedicated hobbyist! I’ll continue to add data as they become available which shouldn’t be long – it seems every month brings forth new information. Questions? Comments? I’m best reached at [email protected]
How to Use This List
There are several approaches in using this list. The first, and perhaps most simple, is applicable to those with an established tank in which specimens are thriving. Any coral with a matching zooxanthellae clade will probably do well within the same aquarium. A more precise, though limited method, requires use of a quantum or PAR meter. Compare the PAR measurement from the corals intended place to the PAR measurements within the list.
The list can be downloaded in PDF format. Please download Adobe Acrobat Reader if you do not have it installed on your system.
Column 1. Animal hosts are most often listed using Latin names – a necessity considering the confusion a list of this sort would generate if common names (i.e., Bali green hairy mushroom) were used. Use of the listing may therefore require some effort on the hobbyist’s part for proper identification (at least to the genus level). Such references are readily available to hobbyists. I have also included clades when they are only casually mentioned in a journal article and no coral host is mentioned (designated as “?”).
Column 2. Practical information is often included about the regional location of the host invertebrate. It is soon realized that Porites coral are pandemic, while some corals are endemic to certain isolated areas (Hawaiian coral species are a good example). Though it is not likely that Hawaiian corals are found in home aquaria, it is possible a zooxanthellae clade is not restricted to the Hawaiian Archipelago, and may be found in host corals from other regions. Therefore this information is of potential use since we have information on photosynthetic saturation levels of some Hawaiian corals.
Abbreviations are: AC = Atlantic Caribbean; C = Caribbean (C, for Caribbean, is also used as a prefix to identify location in countries with Atlantic and Pacific shorelines, i.e., Panama); CC = Central Caribbean; Central GBR = Central Great Barrier Reef, eastern Australia; CP = Central Pacific; EC = Eastern Caribbean; EP = Eastern Pacific; GBR = Great Barrier Reef, eastern Australia; IP = Indo-Pacific Ocean; NC = Northern Caribbean; P-Panama = Pacific shore of Panama; RS = Red Sea; Taiwan-KT = Kenting Island; Taiwan-PI = Penghu Island; WC = Western Caribbean; WI = Western Indian Ocean; and WP = Western Pacific.
Column 3. Truncated comments are included for ease of reference, along with journal references for further study. During review, one will quickly realize how diverse the genus Symbiodinium actually is. Instead of making things more complicated, all this information will begin to make things easier for hobbyists in that trends begin to evolve and, at times, generalizations can be made. These, along with the quality and quantity of rapidly evolving information, will someday precisely answer many of the remaining questions about the lighting requirements of those animals in our captive reefs. Occasionally, I have added some light requirement information, and have made an assumption that a particular subclade (C27, for instance) will have the same range of light needs regardless of location (and will react in the same manner to saturating light intensity within an aquarium). This is based on P/I curves of Hawaiian corals and cross-referenced with light ranges made in the field by researchers referenced below. The saturation numbers listed in this column are full-blown saturation levels (not saturation onset numbers) where increasing light intensity will not increase the rate of photosynthesis. Kirk (1983) recommends saturation onset as the standardized method of reporting photosynthetic saturation. I have chosen otherwise, since coral geometry is often highly irregular and subject to shading. Using full saturation as the standard should ensure that shaded areas have sufficient light.
Column 4. Appropriate journal references. Full information is available below.
References and Further Reading
- Abrego, D., K. Ulstrap, B. Willis, and M. van Oppen, 2008. Species-specific interactions between endosymbionts and coral hosts define their bleaching response to heat and light stress. Proc. Royal Soc. B, 275:2273-2282.
- Baillie, B., C. Belda-Baillie and T. Maruyama, 2000. Conspecificity and Indo-Pacific distribution of Symbiodinium genotypes (Dinophyceae) from giant clams. J. Phycol. 36:1153-1161.
- Baker, A., 2001. Reef corals bleach to survive change. Nature, 401: 765-766.
- ———–, 2003. Flexibility and specificity in coral/algal symbiosis: Diversity, ecology and biogeography of Symbiodinium. Annu. Rev. Ecol. Syst., 34:661-689.
- ———–, In Press. Symbiont diversity on coral reefs and its relationship to bleaching resistance and resilience.
- ————, and R. Rowan, 1997. Diversity of symbiotic dinoflagellates (zooxanthellae) in scleractinian corals of the Caribbean and eastern Pacific. Proc. 8th Int. Coral Reef Symp., Panama. 2: 1301-1306.
- ————-, R. Rowan and N. Knowlton, 1997. Symbiosis ecology of two Caribbean Acroporid corals. Proc. 8th Int. Coral Reef Symp., Panama. 2:1295-1300.
- Banaszak, A.., M. Santos, T. LaJeunesse and M. Lesser, 2006. The distribution of mycosporine-like amino acids (MAAs) and the phylogenetic identity of symbiotic dinoflagellates in cnidarian hosts from the Mexican Caribbean. J. Exp. Mar. Biol. Ecol., 337:131-146.
- ——————, T. LaJeunesse and R. Trench, 2000. The synthesis of mycosporine-like amino acids (MAAs) by cultured, symbiotic dinoflagellates. J. Exp. Mar. Biol. Ecol., 249: 219-233.
- Barneah, O., V. Weis, S. Perez and Y. Benayahu, 2004. Diversity of dinoflagellates symbionts in Red Sea soft corals: Mode of acquisition matters. Mar. Ecol. Prog. Ser., 275: 89-95.
- ————-, I. Brickner, M. Hodge, V. Weiss, T. LaJeunesse, and Y. Benayahu, 2007. Three party symbiosis: Acoelomorph worms, corals and unicellular algal symbionts in Eilat (Red Sea). Mar. Biol.
- Baums, I., M. Johnson, M. Delvin-Durante, and M. Miller, 2010. Host population genetic structure and diversity of two reef-building coral species along the Florida Reef Tract and wider Caribbean. Coral Reefs, 29:835-842.
- Berkelmans, R. and M. van Oppen, 2006. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc. Royal Soc. B., 273:2305-2312.
- Brown, B.E., I. Ambarsari, M.E. Warner, W.K. Fitt, R.P. Dunne, S.W. Gibb and D.G. Cummings, 1999. Diurnal changes in photochemical efficiency and xanthophyll concentrations in shallow water reef corals: evidence for photoinhibition and photoprotection. Coral Reefs, 18:99-105.
- Costa, C., R. Sassi, and K. Gorlach-Lira, 2008. Zooxanthellae genotypes in the coral Siderastrea stellata from coastal reefs in northeastern Brazil. J. Exp. Mar. Biol. Ecol., 367(2):149-152.
- Chen, C., Y-W Yang, N. Wei, W-S Tsai and L-S Fang, 2005. Symbiont diversity in scleractinian corals from tropical reefs and sub-tropical
- Coffroth, M. and S. Santos, 2005. Genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist, 156:19-34.
- ————, D. Poland, E. Petrou, D. Brazeau, and J. Holmberg, 2010. Environmental symbiont acquisition may not be the solution to warming seas for reef-building corals. PlosOne, 5(10):e13258.
- Costa, C., R. Sassi, and F. Amaral, 2005. Annual cycle of symbiotic dinoflagellates from three species of scleractinian corals from coastal reefs of Brazil. Coral Reefs, 24(2): 191-194.
- Crabbe, M., and J. Carlin, 2009. Multiple Symbiodinium clades in Acropora species scleractinian corals from the Ningaloo reef, Australia. Int. J. Integr. Biol., 5(2):72-74.
- Diaz-Almeyda, E., P. Thome, M. El Hafidi, and R. Iglesias-Prieto, 2011. Differential stability of photosynthetic membranes and fatty acid composition at elevated temperature in Symbiodinium. Coral Reefs, 30:217-225.
- Fabricius, K., 2006. Effects of irradiance, flow, and colony pigmentation on the temperature microenvironment around corals: Implications for coral bleaching? Limnol. Oceanogr., 51(1): 30-37.
- ————, J. Mieog, P. Colin, D. Idip, and M. van Oppen, 2004. Identity and diversity of coral reef endosymbionts (zooxanthellae) from three Palauan reefs with contrasting bleaching, temperature and shading histories. Mol. Ecol., 13(8):2445-2458.
- Garren, M., S. Walsh, A. Caccone and N. Knowlton, 2006. Patterns of association between Symbiodinium and members of the Montastraea annularis species complex on spatial scales ranging from within colonies to between geographical regions. Coral Reefs, 25: 503-512.
- Gosliner, T., D. Behrens and G. Williams, 1996. Coral Reef Animals of the Indo-Pacific. Sea Challengers, Monterey, Ca. 314 pp.
- Goulet, T. and M. Coffroth, 2004. The genetic identity of dinoflagellates symbionts in Caribbean octocorals. Coral Reefs, 23: 465-472.
- Grottoli-Everett, A.G. and L.B. Kuffner, 1995. Uneven bleaching within the colonies of the Hawaiian coral Montipora verrucosa. In: Ultraviolet Radiation and Coral Reefs. D. Gulko and P.L. Jokiel, Eds. HIMB Tech. Report #41.
- Hennige, S., D. Suggett, M. Warner and D. Smith, 2006. Photoacclimation of Symbiodinium revisited: Variation of strategies with thermal tolerance? Natural Environment Research Council, University of Essex.
- Hunter, C.L., C.W. Morden, and C.M. Smith, 1997. The utility of ITS sequences in assessing relationships among zooxanthellae and corals. Proc. 8th Int. Coral Reef Symp., Panama. 2: 1599-1602.
- Iglesias-Prieto, R. V. Beltram, T. LaJeunesse, H. Reyes-Bonilla, and P. Thomas, 2004. Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc. R. Soc. Lond. B., 271:1751-1763.
- Jeffrey, S., R. Mantoura and S. Wright, eds., 1997. Monographs on Oceanographic Methodology: Phytoplankton Pigments in Oceanography. UNESCO Publications, Paris. 661 pp.
- Jones, A., R. Berkelmans, M. van Oppen, J. Mieog, and W. Sinclair, 2008. A community change in the algal symbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc. Royal Soc. B, 275:1359-1365.
- Kahng, S. and J. Maragos, 2006. The deepest, zooxanthellate scleractinian corals in the world? Coral Reefs, 25(2):254.
- Kemp, D., C. Cook, T. LaJeunesse and W. Brooks, 2006. A comparison of thermal bleaching responses of the zoanthid Palythoa caribaeorum from three geographically different regions of south Florida. J. Exp. Mar. Biol. Ecol. 335: 266-276.
- Kirk, J.T.O., 1983. Light and Photosynthesis in Aquatic Ecosystems. Cambridge University Press, Cambridge. 401 pp.
- Kirk, N., J. Ward and Coffroth, 2005. Stable Symbiodinium composition in the sea fan Gorgonia ventalina during temperature and disease stress. Biol. Bull., 209: 227-234.
- Kuffner, I.B., M.E. Ondrusek and M.P. Lesser, 1995. Distribution of mycosporine-like amino acids in the tissues of Hawaiian scleractinia: a depth profile. In: Ultraviolet Radiation and Coral Reefs. D. Gulko and P.L. Jokiel, Eds. HIMB Tech. Report #41.
- LaJeunesse, T. and R. Trench, 2000. Biogeography of two species of Symbiodinium (Freudenthal) inhabiting the inter-tidal anemone Anthopleura elegantissima (Brandt). Biol. Bull. 199: 126-134.
- —————–, 2000b. Investigating the biodiversity, ecology and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region in search of a species level marker. J. Phycol., 37: 866-890.
- —————–, 2002. Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar. Biol., 141: 387-400.
- ——————, W. Loh, R. vanWoesik, O. Hoegh-Guldberg, G. Schmidt and W. Fitt, 2003. Low symbionts diversity in southern Great Barrier Reef corals, relative to those in the Caribbean. Limnol. Oceanogr., 48(5):2046-2054.
- —————–, D. Thornhill, E. Cox, F. Stanton, W. Fitt and G. Schmidt, 2004. High diversity and host specificity observed among symbiotic dinoflagellates in reef coral communities from Hawaii. Coral Reefs, 23:596-603.
- ———————-, R. Bhagooli, M. Hidaka, L. de Vantier, T. Done, G. Schmidt, W. Fitt and O. Hoegh-Guldberg, 2004b. Closely related Symbiodinium species differ in relative dominance in coral reef host communities across environmental latitudinal and Biogeographical gradients. Mar. Ecol. Prog. Ser., 284: 147-161.
- ——————, 2005. “Species” radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene-Pliocene transition. Mol. Biol. Evol. 22(3): 570-581.
- ———————, G. Lambert, R. Andersen, M. Coffroth, and D. Galbraith, 2005. Symbiodinium (Phyrhophyta) genome sizes (DNA content) are smallest among dinoflagellates. J. Phycol., 41: 880-886.
- ——————, S. Lee, S. Bush and J. Bruno, 2005b. Persistence of non-Caribbean algal symbionts in Indo-Pacific mushroom corals released to Jamaica 35 years ago. Coral Reefs, 24(1): 157-160.
- ———————, H. Reyes-Bonilla and M. Warner, 2007. Spring ‘bleaching’ among Pocillopora in the Sea of Cortez, Eastern Pacific. Coral Reefs, in press.
- ———————, W. Loh and R. Trench, 2008. Do introduced endosymbiotic dinoflagellates ‘take’ to new hosts? Biol. Invasions.
- ——————–, W. Fitt and G. Schmidt, 2010. The reticulated chloroplasts of zooxanthellae (Symbiodinium) and differences in chlorophyll localization among life cycle stages. Coral Reefs, 29:627.
- ——————–, R. Smith, J. Finney, and H. Oxenford, 2009. Outbreak and persistence of opportunistic Symbiodinium dinoflagellates during the 2005 Caribbean mass coral bleaching event. Proc. Royal Soc. B., 276:4139-4148.
- ——————–, R. Smith, M. Walther, J. Pinzon, D. Pettay, M. McGinley, M. Aschaffenburg, P. Medina-Rosas, A. Cupul-Magana, A. Lopez Perez, H. Reyes-Bonilla, and M. Warner, 2010. Host-symbiont recombination versus natural selection in the response of coral-dinoflagellate symbioses to environmental disturbance. Proc. Royal Soc. B., 277:2925-2934.
- Lesser, M., 1996. Elevated temperatures and ultraviolet radiation cause oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates. Limnol. Oceanogr. 41:271-283.
- Lien, Y.-T., Y. Nakano, S. Plathong, H. Fukami, J.-T. Wang and C. Chen, 2007. Occurrence of the putatively heat-tolerant Symbiodinium phylotype D in high-latitudinal outlying coral communities. Coral Reefs, in press.
- Little, A., M. van Oppen and B. Willis, 2004. Flexibility in algal endosymbioses shapes growth in reef corals. Science, 304(5676):1492-1494.
- Loh, W., T. Loi, D. Carter and O. Hoegh-Guldberg, 2001. Genetic variability of the symbiotic dinoflagellates from the wide ranging coral species Seriatopora hystrix and Acropora longicyathus in the Indo-West Pacific. Mar. Ecol. Prog. Ser., 222: 97-107.
- McClanahan, T., J. Maina, R. Moothien-Pillay, and A. Baker, 2005. Effects of geography, taxa, water flow, and temperature variation on coral bleaching intensity in Mauritius. Mar. Ecol. Prog. Ser., 298: 131-142.
- Muller-Parker, G., J. Pierce-Cravens and B. Bingham, 2007. Broad thermal tolerance of the symbiotic dinoflagellates Symbiodinium muscatinei (Dinophyta) in the sea anemone Anthopleura elegantissima (Cnidaria) from northern latitudes. J. Phycol., 43: 25-31.
- Oliver, T. and S. Palumbi, 2011. Many corals host thermally resistant symbionts in high temperature environment. Coral Reefs, 30:241-250.
- Pochon, X., J. Pawlowski, L. Zaninetti and R. Rowan, 2001. High genetic diversity and relative specificity among Symbiodinium-like endosymbiotic dinoflagellates in soritid foraminiferans. Mar. Biol., 139:1069-1078.
- ————-, T. LaJeunesse, and J. Pawlowski, 2004. Biogeographical partitioning and host specialization among foraminiferan dinoflagellates symbionts (Symbiodinium: Dinophyta). Mar. Biol., 146:17-27.
- ————–, J. Montoya-Burgos, B. Stadelmann and J. Pawlowski, 2005. Molecular phylogeny, evolutionary rates, and divergence timing of the symbiotic dinoflagellates genus Symbiodinium. Molecular Phylogenetics and Evolution, in press.
- Ralph, P., R. Gademan, A. Larkum and M. K002. Spatial heterogeneity in active chlorophyll fluorescence and PSII activity of coral tissues. Mar. Biol., 141: 639-646.
- ———, R. Gademan, A. Larkum and M. K005. Temporal patterns in effective quantum yield of individual zooxanthellae expelled during bleaching. J. Exp. Mar. Biol. Ecol., 316: 17-28.
- Reimer, J., K. Takishita and T. Maruyama, 2006. Molecular identification of symbiotic dinoflagellates (Symbiodinium spp.) from Palythoa spp. (Anthozoa: Hexacorallia) in Japan. Coral Reefs, 25: 521-527.
- Riddle, D., 2004a. Playing with poison: Ultraviolet radiation. Advanced Aquarist Online 3(8), August 2004. http://www.advanceaquarist.com/issues/aug2004/feature.htm
- Riddle, D., 2004b. Too much light! Advanced Aquarist Online 3(7), July 2004. http://www.advanceaquarist.com/issues/july2004/feature.htm
- Riddle, D., 2007. How much light?! Analyses of Selected Shallow Water Invertebrates’ Light Requirements. Advanced Aquarist Online March 2007. http://www.advancedaquarist.com/2007/3/aafeature1/
- Robinson, J. and M. Warner, 2006. Differential impacts of photoacclimation and thermal stress on the photobiology of four phylotypes of symbiotic dinoflagellates. Poster presentation, AGU meeting.
- Rodriguez-Lanetty, M., 2002. Evolving lineages of Symbiodinium-like dinoflagellates based on ITS1 rDNA. Molecular Phylogenetics Evolution, 28: 152-168.
- ———————–, H. Cha, and J. Song, in press. Genetic diversity of symbiotic dinoflagellates associated with anthozoans from Korean waters. Proc. 9th Int. Coral Reef Symp.
- ———————–, and O. Hoegh-Guldberg, 2003. Symbiont diversity within the widespread scleractinian coral Plesiastrea verispora, across the northwestern Pacific. Mar. Biol., 143:501-509.
- ———————–, D. Krupp, and V. Weis, 2004. Distinct ITS types of Symbiodinium in Clade C correlate with cnidarian/dinoflagellate specificity during onset of symbiosis. Mar. Ecol. Prog. Ser., 275:97-102.
- ———————-, W. Loh, D. Carter, and O. Hoegh-Guldberg, 2001. Latitudinal variability in symbiont specificity within the widespread scleractinian coral Plesiastrea verispora. Mar. Biol., 138:1175-1181.
- Rowan, R. and N. Knowlton, 1995. Intraspecific diversity and ecological zonation in coral-algal symbiosis. Proc. Natl. Acad. Sci., USA. 92, 2850-2853.
- Rowan, S. and D.A. Powers, 1993. Molecular genetic comparisons of zooxanthellae from different places. Proc. 7th Int. Coral Reef Symp., Guam. 2: 658. (Abstract).
- Sampayo, E., T. Ridgway, P. Bonagerts, and O. Hoegh-Guldberg. Bleaching suspectibilityand mortality of corals are determined by fine-scale differences in symbiont type. PNAS, 105(30): 10444-10449.
- Santos, S., D. Taylor and M. Coffroth, 2001. Genetic comparisons of freshly isolated versus cultured symbiotic dinoflagellates: Implications to extrapolating to the intact symbiosis. J. Phycol., 37:900-912.
- ———–, T. Shearer, A. Hannes and M. Coffroth, 2004. Fine-scale diversity and specificity in the most prevalent lineage of symbiotic dinoflagellates (Symbiodinium, Dinophyceae) of the Caribbean. Molecular Ecology, 13: 459-469.
- Savage, A., M. Goodson, S. Visram, H. Trapido-Rosenthal, J. Wiedenmann and A. Douglas, 2002. Molecular diversity of symbiotic algae at the latitudinal margins of their distribution: Dinoflagellates of the genus Symbiodinium in corals and anemones. Mar. Ecol. Prog. Ser., 244: 17-26.
- ————–, H. Trapido-Rosenthal and A. Douglas, 2002a. On the functional significance of molecular variation in Symbiodinium, the symbiotic algae of Cnidaria: Photosynthetic response to irradiance. Mar. Ecol. Prog. Ser., 244: 27-37.
- Secord, D. and G. Muller-Parker, 2005. Symbiont distribution along a light gradient within an intertidal cave. Limnol. Oceanogr., 50(1):272-278.
- Shearer, T., C. Gutierez-Rodrigez and M. Coffroth, 2005. Generating molecular markers from zooxanthellate cnidarians. Coral Reefs 24: 57-66.
- Shick, J., W. Dunlap, J. Pearse and V. Pearse, 2002. Mycosporine-like amino acid content in four species of sea anemones in the genus Anthopleura reflects phylogenetic but not environmental or symbiotic relationships. Biol. Bull., 203:315-330.
- Smith, R., J. Pinzon, and T. LaJeunesse, 2009. Aquarium corals under long-term cultivation maintain Symbiodinium spp. found in wild populations. J. Phycol., 45(51):1030-1036.
- Stat, M., D. Carter, and O. Hoegh-Guldberg, 2006. The evolutionary history of symbiont and scleractinian hosts – Symbiosis, diversity and the effect of climate change. Persp. Plant Ecol. Evol. Syst., 8:23-43.
- ——–., E. Morris, and R. Gates, 2008. Functional diversity in coral-dinoflagellate symbiosis. PNAS, 105(27): 9256-9261.
- Strychar, K., M. Coates, P. Sammarco, T. Piva, T. and P. Scott, 2005. Loss of Symbiodinium from bleached soft corals Sarcophyton ehrenbergi, Sinularia sp and Xenia sp.. J. Exp. Mar. Biol. Ecol., 320 2: 159-177.
- Suggett, D., M. Warner, D. Smith, P. Davey, S. Hennige, and N. Baker, 2008. Photosynthesis and production of hydrogen peroxide by Symbiodinium (Pyrrhophyta) phylotypes with different thermal tolerances. Phycol. Soc. America.
- Takabayashi, M., S. Santos and C. Cook, 2004. Mitochondrial DNA phylogeny of the symbiotic dinoflagellates (Symbiodinium, Dinophyta). J. Phycol., 40, 160-164.
- Tchernov, D., M. Gorbunov, C. de Vargas, S. Yadav, A. Milligan, M. Hublom, and P. Falkowski, 2004. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc. Natl. Acad. Sci. USA, 101, 37: 13531-13535.
- Thornhill, D. T. LaJeunesse, D. Kemp, W. Fitt and G. Schmidt, 2005. Multiyear seasonal genotypic survey of coral-algal symbioses reveals prevalent stability or post-bleaching reversion. Mar. Biol.
- Thornhill, D., W. Fitt and G. Schmidt, 2006. Highly stable symbioses among western Atlantic brooding corals. Coral Reefs, 25: 515-519.
- ————, D. Kemp, B. Bruns, W. Fitt and G. Schmidt, 2008. Correspondence between cold tolerance and temperate biogeography in a western Atlantic Symbiodinium (Dinophyta) lineage. J. Phycol., 44:1126-1135.
- Trench, R.K., 1997. Diversity of symbiotic dinoflagellates and the evolution of microalgal-invertebrate symbioses. Proc. 8th Int. Coral Reef Symp., Panama. 2: 1275-1286.
- Ulstrap, K. and M. van Oppen, 2003. Geographic and habitat partitioning of genetically distinct zooxanthellae (Symbiodinium) in Acropora corals on the Great Barrier Reef. Mol. Ecol., 12(12): 3477-3484.
- ———-, P. Ralph, A. Larkum and M. Kuhl, 2006. Intra-colonial variability in light acclimation of zooxanthellae in coral tissues of Pocillopora damicornis. Mar. Biol. 149: 1325-1335.
- Van Oppen, M., 2004. Mode of zooxanthella transmission does not affect zooxanthella diversity in Acroporid corals. Mar. Biol., 144: 1-7.
- ——————, F. Palstra, A. Piquet and D. Miller, 2001. Patterns of coral-dinoflagellate associations in Acropora: Significance of local availability and physiology of Symbiodinium strains and host-symbiont selectivity. Proc. R. Soc. Lond B., 268: 1759-1767.
- ——————-, J. Mieog, C. Sanchez, and K. Fabricius, 2005. Diversity of algal endosymbionts (zooxanthellae) in tropical octocorals: the roles of geography and host relationships. Mol. Ecol., 14: 2403-2417.
- ——————–, A. Mahiny, and T. Done, 2005b. Geographical distribution of zooxanthella types of three coral species of the Great Barrier Reef sampled after the 2002 bleaching events. Coral Reefs, in press.
- Venera-Ponton, D., G. Diaz-Pulido, M. Rodriguez-Lanetty and O. Hoegh-Guldbery, 2010. Presence of Symbiodinium spp. in microalgal microhabitats from the southern Great Barrier Reef. Coral Reefs, 29:1049-1060.
- Venn, A., J. Loram, H. Trapido-Rosenthal, D. Joyce and A. Douglas, 2008. Importance of time and place: Patterns in abundance of Symbiodinium Clades A and B in the tropical sea anemone Condylactis gigantea. Biol. Bull., 215:243-252.
- Visram, S. and A. Douglas, 2006. Molecular diversity of symbiotic algae (zooxanthellae) in scleractinian corals of Kenya. Coral Reefs, 25: 172-176.
- Vollmer, S. and S. Palumbi, 2004. Testing the utility of internally transcribed spacer sequences in coral phylogenetics. Mol. Ecol., 13: 2763-2772.
- Wagner, D., X. Pochon, L. Irwin, R. Toonen, and R. Gates, 2010. Azooxanthellate? Most Hawaiian black corals contain Symbiodinium. Proc. Royal Soc. B. Published online: doi: 10.1098/rspb.2010.1681
- Wakefield, T. and S. Kempf, 2001. Development of host- and symbiont-specific monoclonal antibodies and confirmation of the origin of the symbiosome membrane in a cnidarian-dinoflagellate symbiosis. Biol. Bull., 200: 127-143.
- Warner, M., T. LaJeunesse, J. Robinson and R. Thur, 2006. The ecological distribution and comparative photobiology of symbiotic dinoflagellates from coral reefs in Belize: Potential implications for coral bleaching. Limnol. Oceanog., 51(4): 1887-1897.
- —————-, and S. Berry-Lowe, 2006. Differential xanthophyll cycling and photochemical activity in symbiotic dinoflagellates in multiple locations of three species of Caribbean corals. J. Exp. Mar. Biol. Ecol., 339: 86-95.
- Wicks, L., E. Sampayo, J. Gardner, and S. Davy, 2010. Local endemicity and high diversity characterize high-latitude coral-Symbiodinium partnerships. Coral Reefs, 29: 989-1003.
- ———–, R. Hill and S. Davy, 2010. The influence of irradiance on tolerance to high and low temperature stress exhibited by Symbiodinium in the coral Pocillopora damicornis from the high-latitude reef of Lord Howe Island. Limnol. Oceanogr., 55(6):2476-2486.



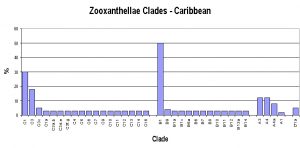


0 Comments