We often see articles in hobby literature referring to locations where corals and other photosynthetic invertebrates are collected. These articles are informative, interesting and offer a perspective of the environmental conditions required for coral growth. But what if we could look at requirements, particularly at genetically encoded requirements, of the symbiotic dinoflagellates zooxanthellae within corals tissues? Though genetic fingerprinting of zooxanthellae is very much in its infancy, and with only limited (but rapidly expanding) information from researchers, we, as hobbyists, can begin to accurately piece together some zooxanthellae – and hence corals – specific requirements for successful maintenance in captivity. By extension, we can predict the lighting environment and physical conditions required by other zooxanthellae/corals.
A need for information on zooxanthellae found within the tissues of Hawaiian corals began innocently enough. The information I required was in a recent issue of Coral Reefs, however I became curious about other available data. I checked my available references and began tracking down their cited journal articles, and the effort quickly became geometric. I did not realize that so much research had recently been conducted. My little project soon blossomed into a time-consuming venture. The compiled database (presented in this article) lists symbiotic corals (stony and soft – often to the species level), mollusks, false corals, anemones, flatworms, and hydrocorals along with pertinent information. With over 800 entries of species and variations of subspecies (called clades), I believe this to be the most complete listing readily available to hobbyists.
Why should anyone be interested in a rather obscure subject such as this? After all, we know that coral animals (hosts) and zooxanthellae (symbionts) have a mutually beneficial relationship. We realize zooxanthellae need light and either too much, or not enough, photosynthetically active radiation will cause problems. In the most severe cases, the coral animal will eject its zooxanthellae in a process known as bleaching. Bleaching is generally exacerbated by higher than normal water temperature and ultraviolet radiation. This information is elementary. It gets more involved. Much more involved.
The compiled database (presented in this article) lists symbiotic corals (stony and soft – often to the species level), mollusks, false corals, anemones, flatworms, and hydrocorals along with pertinent information. With over 800 entries of species and variations of subspecies (called clades), I believe this to be the most complete listing readily available to hobbyists.
Advances in DNA fingerprinting have allowed researchers to identify many life forms to species and subspecies level. A handful of dedicated scientists are devoting their careers to the investigation of various types of zooxanthellae, and are generating a great deal of data. We’ve known for some time that there isnt just a single species of zooxanthellae (Symbiodinium microadriaticum). We now know there are at least 9 described species with many subspecies (variously called clades, types or phylotypes). There are Clades A, B, C, D, E, F, G and H. Of these, Clades A, B, C, D (and to a lesser degree F and G) are of most interest to reef aquaria hobbyists (see Figure 1). Symbiont populations tend to follow Fisher log-normal distribution patterns characterized by ‘generalist’ zooxanthellae (common) and rare zooxanthellae (‘specialists’) hosted by specific coral species (Pochon et al., 2001). For instance, some zooxanthellae clades are tolerant of high light intensity, while others have higher thermal tolerances, and this is where it begins to get interesting to hobbyists.
Each Clade contains sub-clades, and variations of sub-clades. In the following database, zooxanthellae sub-clades are designated by a numeral following the clade and, in some cases, a lower case letter for further refinement (i.e., C1a indicates Clade C, and the lower case letter indicates a variation of subclade 1). The following listing reports more than 150 of them. Information is also listed for accession numbers this is a code assigned to genetic fingerprints in GenBanks database. I have not attempted to distinguish between ITS (internal transcribed spacer, only recently applied to zooxanthellae Hunter et al., 1997) and any other methods accession number, although its a good bet that most definitions beyond the clade level (in this list anyway) were performed by fingerprinting the ITS region (a less conserved portion of the rDNA). The accession numbers were included in the original Excel file (available upon request) in order to sort by GenBanks codes and determine common host/symbiont relationships. For those really serious about further information, a web search on a particular accession number will occasionally turn up additional information.
Even with advances in DNA fingerprinting, there is a question of speciation. What genetic markers determine if a certain clade of zooxanthellae rises to the level of becoming a new species? Until integrated examinations are completed, these questions will remain unresolved (Takabayashi et al, 2004). This isnt that much of an issue as far as hobbyists are concerned but it is causing constant revisions in the taxonomy of symbiotic dinoflagellates.
However, there is general not universal – agreement about zooxanthellae clades. Atlantic and Caribbean corals usually contain variations of Clade B (there are exceptions of course!) with A and C making up the difference. On the other hand, Pacific corals usually contain variations of Clade C (again with exceptions to the rule). It is believed that closure of the Central American seaway by the rise of the isthmus that is now Central America created distinct zones for coral growth and zooxanthellae specialization. The survival of Atlantic corals during glaciation of the northern hemisphere (the Ice Age) depended upon adaptation resulting in co-dominant zooxanthellae clades, while Pacific corals enjoyed mostly tropical environs during this period and C clades dominated.
Clades of Zooxanthellae
As mentioned earlier, taxonomy of zooxanthellae is constantly revised, with elevation from clade level to species not particularly uncommon. In addition, there is confusion created by using cultures of zooxanthellae – Santos et al. (2001) report that zooxanthellae cultured in vitro may not be representative of the dominant in hospite zooxanthellae clade since conditions within the culture vessel may favor the growth of a sub-dominant clade. This is an important point to consider when reviewing early research works. However, this list is believed to be correct as of late 2005.
I have included zooxanthellae species under the appropriate clade heading. Trends begin to develop and suggest (but do not confirm) characteristics that are perhaps common to each particular zooxanthellae clade.
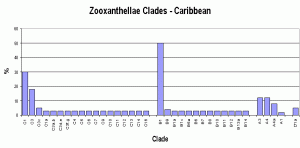
For a general distribution map of clades, see Figure 2. For populations within a given region, see Figures 3 and 4.
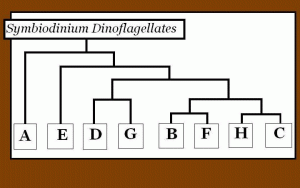
Figure 1. A gene tree of zooxanthellae clades showing how different clades are related to each another. This is a synthesis of works performed by Coffroth and Santos (2005), and is based on ITS analyses.
Traits of Different Clades and Why They Are Important
As mentioned, we begin to see traits common among zooxanthellae clades. Two important traits are Xanthophyll Production and production of Mycosporine-like Amino Acids (MAAs). Again, assigning characteristics found in one clade species to all zooxanthellae found within a particular clade is risky business. However, trends do seem to develop upon close examination, at least in Clade A and Clade B.
Xanthophylls are carotenoid pigments found within many species of zooxanthellae, algae and higher plants. The two xanthophylls found in some zooxanthellae are diadinoxanthin (Dn) and diatoxanthin (DT) Brown et al., 1999. Dn and DT act as a photoprotectants and shield those zooxanthellae containing them from excessively high amounts of photosynthetically active radiation in a process called Dynamic Photoinhibition. This is simply a protective measure that prevents damage to Photosystem II. In high light, Dn absorbs blue wavelengths (Jeffries, 1997) and is converted to DT, thus shunting blue light energy away from the photosynthetic apparatus. Zooxanthellae with the ability to produce xanthophylls are equipped to endure higher light intensities with a lessened chance of destruction of their light harvesting proteins. (It should be noted that other energy dissipation pathways may be available such as release of non-radiant heat by the Photosystem II Reaction Center, or perhaps spillover of energy from Photosystem II to Photosystem I.)
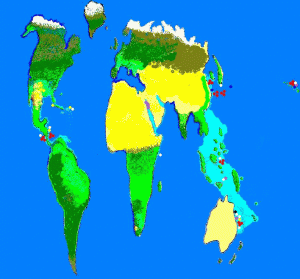
Figure 2. Known locations of zooxanthellae clades. Purple = Clade A; Yellow = Clade B; White = Clade C; Red = Clade D; Dark Blue =Clade F and Black = Clade G. Not surprisingly, identified clade locations are usually near research stations.
While xanthophylls protect zooxanthellae from visible light energy, mycosporine-like amino acids (MAAs) protect them from ultraviolet radiation. So named because these amino acids were first isolated from fungi, MAAs are produced by plants, fungi and some bacteria. The chemical pathway leading to MAA production (the shikimate pathway) is not known to occur in animals, so MAAs can be obtained from zooxanthellae known to produce them. MAAs can also be obtained through dietary means (ingestion of algae or animals containing accumulated MAAs). Interestingly, Shick et al. report that the temperate sea anemone Anthopleura
elegantissima obtains certain MAAs from ocular lenses in fishes it ingests. It is possible that bacteria and/or cyanobacteria can translocate MAAs, or modify translocated or ingested MAAs. It is also possible that translocated MAAs could be modified by the host coral). In short, MAAs can be obtained from sources other than zooxanthellae. However the ability to produce and release these important compounds to the coral host likely gives the coral a competitive edge in shallow environments.
Clade “A”
Clade A zooxanthellae are generally considered relatively hardy, and are found in scleractinian corals, octocorals, hydrocorals, clams, anemones and zoanthids. Most hosts of Clade A zooxanthellae are found in the Caribbean, with sporadic reports of occurrences in Australias Great Barrier Reef, the Red Sea and the western Pacific (Korea).
Reported Species in Clade A
Symbiodinium microadriaticum. Found within tissues of the jellyfish Cassiopeia xamachana. This zooxanthella species acclimates to high and low light levels and synthesizes natural ultraviolet radiation sunscreens mycosporine-like amino acids or MAAs – (even in the absence of UV), but has low tolerance of temperature swings. Protective xanthophylls are produced in super-saturating light intensities (this light intensity = 250 molmsec; Iglesias-Prieto and Trench, 1997). Considering that S. microadriaticum is found within a motile host and subject to rapidly fluctuating environmental conditions, it is not surprising that this species is tolerant of a wide variety of parameters.
Symbiodinium pilosum. Found in the Caribbean zoanthid Zoanthus sociatus. These are high light adapted (they respond poorly to low light levels), tolerate high temperatures swings and are able to produce and incorporate protective xanthophylls into chlorophyll protein complexes. Iglesias-Prieto and Trench, 1997, found this zooxanthella to be the least adaptive in respect to light intensity of 6 zooxanthellae examined (high light is tolerated while low light intensity is not).
Symbiodinium meandrinae. This zooxanthella was discovered within the tissues of the Atlantic stony coral Meandrina meandrites. Banaszak et al., 2000, found two zooxanthellae clades (A and C) within M. meandrites, Baker and Rowan (1997) report Clade B. This leads to confusion over the actual identity of S. meandrinae Trench (1997) clarifies the situation by listing S. meandrinae as Clade A.
Symbiodinium corculorum. Isolated from the photosynthetic Pacific clam Corculorum cardissa. Iglesias-Prieto and Trench (1997) suggest this zooxanthella species has limited photoacclimation capability and the symbiont/host perform best under high light intensity. This clam to limited to a depth of 10 meters (Gosliner et al., 1996) and is thus considered tolerant of high light.
Symbiodinium cariborum. Found in the tissues of the Caribbean anemone Condylactis gigantea.
Summary: Clade A zooxanthellae seem tolerant of high light intensity, and likely produce protective xanthophylls and mycosporine-like amino acids.
Clade “B”
As with Clade A zooxanthellae, those of Clade B are relatively resistant to bleaching episodes. Current information suggests this clade is most common in Caribbean octocorals (sea fans, sea whips, etc.), but also present in many (a dozen or more) Atlantic stony coral genera and at least 8 Acropora species from the Great Barrier Reef. A subclade (B1) has been found in Hawaiian Aiptasia anemones and stony coral Pocillopora damicornis (probably as a cryptic symbiont Santos et al., 2004).
Reported Species in Clade B
Symbiodinium pulchrorum. Found in the Hawaiian anemone Aiptasia. Iglesias-Prieto and Trench (1997) report S. pulchrorum has a high photoacclimatory capability (their experiment used 40 molmsec as the sub-saturating intensity, and 250 molmsec as the super-saturating light intensity). Banaszak (2000) did not find this species to synthesize MAAs. As a footnote to these observations, I have noticed that Aiptasia anemones do not fair well under high light intensity – they retract into small blobs, probably in an effort to self-shade their zooxanthellae from high PPFD (600 molmsec and higher) and/or UV radiation.
Symbiodinium bermudense. A symbiont of the pest anemone Aiptasia pallida. This species apparently does not produce MAAs (Banaszak et al., 2000).
Symbiodinium muscatinei. This species has been described as found in tissues of the temperate anemone Anthopleura elegantissima. It is thought that this species does not produce UV sunscreens (mycosporine-like amino acids, Shick et al., 2002). S. muscatinei is sometimes listed as Clade E. (Santos et al., 2001). Secord and Muller-Parker (2005) found that S. muscatinei and S. californium are tolerant of high light intensity and photosynthetic saturation was not achieved at 540 molmsec. The compensation point for these algae was about 73 molmsec.
Symbiodinium californium. This species does not produce mycosporine-like amino acids in culture (in Shick et al., 2002). It is found within the Anthopleura elegantissima anemone. S. californium is sometimes listed as Clade E (Santos et al., 2001).
Summary for Clade B zooxanthellae species: Do not seem to synthesize mycosporine-like amino acids, and are tolerant of higher light intensities.
Clade “C”
Clade C is difficult to characterize, though Atlantic Clade C zooxanthellae are found in deeper water, while bleaching is often noted in Pacific corals containing Clade C symbionts. Generally, most Clade C zooxanthellae/corals inhabit tropical latitudes.
Clade C contains over 130 subclades, and, as a group, is pandemic. It is found in some Caribbean corals, but most often in the Pacific.
Some Clade Cs are thermally-tolerant (C15), others are generalists exhibiting habitation over a broad range of depths (C1, C3 and C21), C8a is found only in deeper waters, C7c is limited to relatively shallow depths and in nature tolerates light intensity up to about 700 molmsec. It is easy to see why Tchernov et al., 2004 warn of assuming closely related sister subclades will demonstrate similar traits (light and/or temperature tolerances for example).
Reported Species in Clade C
Symbiodinium goreaui. Found within Ragactis lucida (in Trench, 1996) and expanded by LaJeunesse et al., 2003 to the pandemic generalist zooxanthellae Clade C1.
Clade D
Phylotype “D” Relatively resistant to bleaching (in comparison to many Clade C phylotypes), and, in fact, often found in areas that have suffered recent, severe bleaching episodes and hot environments. Chen et al., 2003, found this clade within high latitude corals Oulastrea crispata and Goniastrea aspera inhabiting marginal sites (extreme temperatures, turbidity and irradiance) This zooxanthellae is thus considered extremely stress tolerant. Clade D is the proper classification for symbionts listed in earlier works by Carlos et al. (1999) and Toller (2001 a, b).
Clade E
This clade is not known to occur in corals. Those zooxanthellae listed as Clade E in Toller et al., (2001) have been reclassified as Clade D. Symbiodinium muscatinei
and S. californium (from the anemone Anthopleura) are sometimes listed as belonging to Clade E; they are listed as Clade B (above).
Clade “F”
Normally found in foraminiferans, researchers were surprised when Clade Fr2 was found in isolated ‘daisy coral’ specimens (Alveopora japonica) in Korea. (Rodriguez-Lanetty et al., 2000). Clade F5 occurs in Montipora capitata. F5 is not tolerant of high light intensity, but there are reports of M. capitata containing MAAs. It is not known if these are obtained through diet or translocation.
Reported Species in Clade F
Symbiodinium kawagutii. This zooxanthella species (designated as Clade F5) is found within the Hawaiian coral Montipora capitata (formerly M. verrucosa). No protective xanthophylls are produced as a response to super-saturating irradiance (Iglesias-Prieto and Trench, 1997), and this zooxanthella (and host) does poorly in high light intensity. It is interesting that both corals containing Clade F are found at higher latitudes.
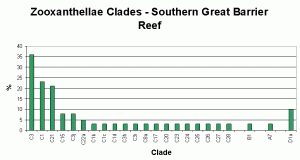
Figure 4. Great Barrier Reef zooxanthellae population by clade. From LaJeunesse, 2003. This could be modified to include Clade G.
Clade G
Clade G has recently been found in soft corals (van Oppen, 2005a), stony corals (van Oppen 2005b) and giant sea anemones (LaJeunesse, in Pochon, 2005).
Why Are Some Zooxanthellae Resistant to Bleaching?
This question begs an answer why do some corals perform better than others at higher light intensity and/or temperatures and seem immune from the effects of radiation? There are many reasons why a coral could be resistant to bleaching:
- Protection from UV Radiation. As we have seen, some zooxanthellae are able to protect themselves from ultraviolet radiation by production of mycosporine-like amino acids. Others can not produce these protectants, and hobbyists have no way of predicting which corals (or other animals for that matter) may be harmed by UV. See Riddle 2004a for reasons why we should eliminate ultraviolet radiation from aquaria.
- Protection from Intense Light. Some zooxanthellae are able to produce and incorporate xanthophylls to protect themselves from high light intensity. Not all do, and there are alternative protective pathways such as spillover or non-radiant heat dissipation once absorbed light energy enters the reaction center of Photosystem II (See Riddle, 2004b fordetails of high light intensity on captive corals). However some zooxanthellae apparently possess little, if any, means of coping with high light intensity. They will either do well in darker environments or merely survive in a hostile environment.
- Thylakoid Membrane Composition. Recent research suggests even more strategies to resist bleaching. Tchernov (2004) suggest the lipid saturation of the hydrophilic thylakoid membrane within the chloroplast determines resistance to compromise. In effect, the very composition of the light-collecting apparatus predetermines resistance to photodestruction and bleaching.
- Absorption of Heat. A newer paper by Fabricius (2006) found that darker pigmented corals can potentially gain radiant heat and become warmer than the surrounding water temperature. Obviously this could make the zooxanthellae potentially more susceptible to a bleaching event (this happens in aquaria too – Riddle, in press
How to Use This List
There are several approaches in using this list. The first, and perhaps most simple, is applicable to those with an established tank in which specimens are thriving. Any coral with a matching zooxanthellae clade will probably do well within the same aquarium. A more precise, though limited method, requires use of a quantum or PAR meter. Compare the PAR measurement from the corals intended place to the PAR measurements within the list.
The list can be downloaded in PDF format. Please download Adobe Acrobat Reader if you do not have it installed on your system.
Column 1 contains accession numbers. These are of little practical use to hobbyists, with the exception that they are a researchable data point. I have included them for that reason only.
Column 2. Animal hosts are most often listed using Latin names – a necessity considering the confusion a list of this sort would generate if common names (i.e., Bali green hairy mushroom) were used. Use of the listing may therefore require some effort on the hobbyists part for proper identification (at least to the genus level). Such references are readily available to hobbyists. I have also included clades when they are only casually mentioned in a journal article and no coral host is mentioned (designated as ?).
Column 3. Practical information is often included about the regional location of the host invertebrate. It is soon realized that Porites coral are pandemic, while some corals are endemic to certain isolated areas (Hawaiian coral species are a good example). Though it is not likely that Hawaiian corals are found in home aquaria, it is possible a zooxanthellae clade is not restricted to the Hawaiian Archipelago, and may be found in host corals from other regions. Therefore this information is of potential use since we have information on photosynthetic saturation levels of some Hawaiian corals.
Abbreviations are: AC = Atlantic Caribbean; C = Caribbean (C, for Caribbean, is also used as a prefix to identify location in countries with Atlantic and Pacific shorelines, i.e., Panama); CC = Central Caribbean; Central GBR = Central Great Barrier Reef, eastern Australia; CP = Central Pacific; EC = Eastern Caribbean; EP = Eastern Pacific; GBR = Great Barrier Reef, eastern Australia; IP = Indo-Pacific Ocean; NC = Northern Caribbean; P-Panama = Pacific shore of Panama;
RS = Red Sea; Taiwan-KT = Kenting Island; Taiwan-PI = Penghu; WC = Western Caribbean; WI = Western Indian Ocean; and WP = Western Pacific.
Column 4. Where available, collection depths or ranges (in meters) are listed for host animals. We will explore why this information, when taken at face value, is of limited use in determining lighting requirements for corals (depth preferences may be due to skeletal strength and other factors). It is, however, useful for estimating the range of light tolerances of zooxanthellae clades when combined with other information. Note: One reference lists a maximum depth of 90 meters I suspect this is a typo (perhaps 90 feet) and would not interpret it literally.
Column 5. Truncated comments are included for ease of reference, along with journal references for further study. During review, one will quickly realize how diverse the genus Symbiodinium actually is. Instead of making things more complicated, all this information will begin to make things easier for hobbyists in that trends begin to evolve and, at times, generalizations can be made. These, along with the quality and quantity of rapidly evolving information, will some day precisely answer many of the remaining questions about the lighting requirements of those animals in our captive reefs.Occasionally, I have added some light requirement information, and have made an assumption that a particular subclade (C27, for instance) will have the same range of light needs regardless of location (and will react in the same manner to saturating light intensity within an aquarium). This is based on P/I curves of Hawaiian corals and cross-referenced with light ranges made in the field by researchers referenced below. The saturation numbers listed in this column are full-blown saturation levels (not saturation onset numbers) where increasing light intensity will not increase the rate of photosynthesis. Kirk (1983) recommends saturation onset as the standardized method of reporting photosynthetic saturation. I have chosen other wise, since coral geometry is often highly irregular and subject to shading. Using full saturation as the standard should ensure that shaded areas have sufficient light.
Column 6. Appropriate journal references. Full information is available below.
References
- Baillie, B., C. Belda-Baillie and T. Maruyama, 2000. Conspecificity and Indo-Pacific distribution of Symbiodinium genotypes (Dinophyceae) from giant clams. J. Phycol. 36:1153-1161.
- Baker, A., 2001. Reef corals bleach to survive change. Nature, 401: 765-766.
- Baker, A., 2003. Flexibility and specificity in coral/algal symbiosis: Diversity, ecology and biogeography of Symbiodinium. Annu. Rev. Ecol. Syst., 34:661-689.
- Baker, A., In Press. Symbiont diversity on coral reefs and its relationship to bleaching resistance and resilience.
- Baker, A.C. and R. Rowan, 1997. Diversity of symbiotic dinoflagellates (zooxanthellae) in scleractinian corals of the Caribbean and eastern Pacific. Proc. 8th Int. Coral Reef Symp., Panama. 2: 1301-1306.
- Baker, A.C., R. Rowan and N. Knowlton, 1997. Symbiosis ecology of two Caribbean Acroporid corals. Proc. 8th Int. Coral Reef Symp., Panama. 2:1295-1300.
- Banaszak, A., T. LaJeunesse and R. Trench, 2000. The synthesis of mycosporine-like amino acids (MAAs) by cultured, symbiotic dinoflagellates. J. Exp. Mar. Biol. Ecol., 249: 219-233.
- Baker, A., R. Rowan and N. Knowlton, 1997. Symbiosis ecology of two Caribbean Acroporid corals. Proc. 8th Int. Coral Reef Symp., 2:1295-1300.
- Baker, A.C., R. Rowan, 1997. Diversity of symbiotic dinoflagellates (zooxanthellae) in scleractinian corals of the Caribbean and eastern Pacific. Proc. 8th Int. Coral Reef Symp., Panama. 2: 1301-1306.
- Brown, B.E., I. Ambarsari, M.E. Warner, W.K. Fitt, R.P. Dunne,S.W. Gibb and D.G. Cummings, 1999. Diurnal changes in photochemical efficiency and xanthophyll concentrations in shallow water reef corals: evidence for photoinhibition and photoprotection. Coral Reefs, 18:99-105.
- Chen, C., Y-W Yang, N. Wei, W-S Tsai and L-S Fang, 2005. Symbiont diversity in scleractinian corals from tropical reefs and sub-tropical non-reef communities in Taiwan. Coral Reefs, 24(1): 11-22.
- Coffroth, M. and S. Santos, 2005. Genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist, 156:19-34.
- Costa, C., R. Sassi, and F. Amaral, 2005. Annual cycle of symbiotic dinoflagellates from three species of scleractinian corals from coastal reefs of Brazil. Coral Reefs, 24(2): 191-194.
- Fabricius, K., 2006. Effects of irradiance, flow, and colony pigmentation on the temperature microenvironment around corals: Implications for coral bleaching? Limnol. Oceanogr., 51(1): 30-37.
- Gosliner, T., D. Behrens and G. Williams, 1996. Coral Reef Animals of the Indo-Pacific. Sea Challengers, Monterey, Ca. 314 pp.
- Goulet, T. and M. Coffroth, 2004. The genetic identity of dinoflagellates symbionts in Caribbean octocorals. Coral Reefs, 23: 465-472.
- Grottoli-Everett, A.G. and L.B. Kuffner, 1995. Uneven bleaching within the colonies of the Hawaiian coral Montipora verrucosa. In: Ultraviolet Radiation and CoralReefs. D. Gulko and P.L. Jokiel, eds. HIMB Tech. Report #41.
- Hunter, C.L., C.W. Morden, and C.M. Smith, 1997. The utility of ITS sequences in assessing relationships among zooxanthellae and corals. Proc. 8th Int. Coral Reef Symp., Panama. 2: 1599-1602.
- Jeffrey, S., R. Mantoura and S. Wright, eds., 1997. Monographs on Oceanographic Methodology: Phytoplankton Pigments in Oceanography. UNESCO Publications, Paris. 661 pp.
- Kirk, J.T.O., 1983. Light and Photosynthesis in Aquatic Ecosystems. Cambridge University Press, Cambridge. 401 pp.
- Kuffner, I.B., M.E. Ondrusek and M.P. Lesser, 1995. Distribution of mycosporine-like amino acids in the tissues of Hawaiian scleractinia: a depth profile. In: Ultraviolet Radiation and Coral Reefs. D. Gulko and P.L. Jokiel, eds. HIMB Tech. Report #41.
- Iglesias-Prieto, R., V. Beltrn, T. LaJeunesse, H. Reyes-Bonilla and P. Thom, 2004. Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc. R. Soc. Lond. B, 271:1757-1763.
- LaJeunesse, T., S. Lee, S. Bush and J. Bruno, 2005. Persistence of non-Caribbean algal symbionts in Indo-Pacific mushroom corals released to Jamaica 35 years ago. Coral Reefs, 24(1): 157-160.
- LaJeunesse, T., W. Loh, R. vanWoesik, O. Hoegh-Guldberg, G. Schmidt and W. Fitt, 2003. Low symbionts diversity in southern Great Barrier Reef corals, relative to those in the Caribbean. Limnol. Oceanogr., 48(5):2046-2054.
- LaJeunesse, T., D. Thornhill, E. Cox, F. Stanton, W. Fitt and G. Schmidt, 2004. High diversity and host specificity observed among symbiotic dinoflagellates in reef coral communities from Hawaii. Coral Reefs, 23:596-603.
- Little, A., M. van Oppen and B. Willis, 2004. Flexibility in algal endosymbioses shapes growth in reef corals. Science, 304(5676):1492-1494.
- Loh, W., T. Loi, D. Carter and O. Hoegh-Guldberg, 2001. Genetic variability of the symbiotic dinoflagellates from the wide ranging coral species Seriatopora hystrix and Acropora longicyathus in the Indo-West Pacific. Mar. Ecol. Prog. Ser., 222: 97-107.
- Pochon, X., J. Pawlowski, L. Zaninetti and R. Rowan, 2001. High genetic diversity and relative specificity among Symbiodinium-like endosymbiotic dinoflagellates in soritid foraminiferans. Mar. Biol., 139:1069-1078.
- Pochon, X., T. LaJeunesse, and J. Pawlowski, 2004. Biogeographical partitioning and host specialization among foraminiferan dinoflagellates symbionts (Symbiodinium: Dinophyta). Mar. Biol., 146:17-27.
- Pochon, X., J. Montoya-Burgos, B. Stadelmann and J. Pawlowski, 2005. Molecular phylogeny, evolutionary rates, and divergence timing of the symbiotic dinoflagellates genus Symbiodinium. Molecular Phylogenetics and Evolution, in press.
- Riddle, D., 2004a. Playing with poison: Ultraviolet radiation. Advanced Aquarist Online 3(8), August 2004. (http://www.advanceaquarist.com/issues/aug2004/feature.htm)
- Riddle, D., 2004b. Too much light! Advanced Aquarist Online 3(7), July 2004. (http://www.advanceaquarist.com/issues/july2004/feature.htm)
- Rowan, R. and N. Knowlton, 1995. Intraspecific diversity and ecological zonation in coral-algal symbiosis. Proc. Natl. Acad. Sci., USA. 92, 2850-2853.
- Rowan, S. and D.A. Powers, 1993. Molecular genetic comparisons of zooxanthellae from different places. Proc. 7th Int. Coral Reef Symp., Guam. 2: 658. (Abstract).
- Santos, S., D. Taylor and M. Coffroth, 2001. Genetic comparisons of freshly isolated versus cultured symbiotic dinoflagellates: Implications to extrapolating to the intact symbiosis. J. Phycol., 37:900-912.
- Santos, S., T. Shearer, A. Hannes and M. Coffroth, 2004. Fine-scale diversity and specificity in the most prevalent lineage of symbiotic dinoflagellates (Symbiodinium, Dinophyceae) of the Caribbean. Molecular Ecology, 13: 459-469.
- Secord, D. and G. Muller-Parker, 2005. Symbiont distribution along a light gradient within an intertidal cave. Limnol. Oceanogr., 50(1):272-278.
- Shearer, T., C. Gutirrez-Rodrguez and M. Coffroth, 2005. Generating molecular markers from zooxanthellate cnidarians. Coral Reefs 24: 57-66.
- Shick, J., W. Dunlap, J. Pearse and V. Pearse, 2002. Mycosporine-like amino acid content in four species of sea anemones in the genus Anthopleura reflects phylogenetic but not environmental or symbiotic relationships. Biol. Bull., 203:315-330.
- Takabayashi, M., S. Santos and C. Cook, 2004. Mitochondrial DNA phylogeny of the symbiotic dinoflagellates (Symbiodinium, Dinophyta). J. Phycol., 40, 160-164.
- Tchernov, D., M. Gorbunov, C. de Vargas, S. Yadav, A. Milligan, M. Hggblom, and P. Falkowski, 2004. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc. Natl. Acad. Sci. USA, 101, 37: 13531-13535.
- Trench, R.K., 1997. Diversity of symbiotic dinoflagellates and the evolution of microalgal-invertebrate symbioses. Proc. 8th Int. Coral Reef Symp., Panama. 2: 1275-1286.
- Ulstrap, K. and M. van Oppen, 2003. Geographic and habitat partitioning of genetically distinct zooxanthellae (Symbiodinium) in Acropora corals on the Great Barrier Reef. Mol. Ecol., 12(12): 3477-3484.
- Van Oppen, M., F. Palstra, A. Piquet and D. Miller, 2001. Patterns of coral-dinoflagellate associations in Acropora: Significance of local availability and physiology of Symbiodinium strains and host-symbiont selectivity. Proc. R. Soc. Lond B., 268: 1759-1767.
- Van Oppen, M., J. Mieog, C. Snchez, and K. Fabricius, 2005a. Diversity of algal endosymbionts (zooxanthellae) in tropical octocorals: the roles of geography and host relationships. Mol. Ecol., in press.
- Van Oppen, M., A. Mahiny, and T. Done, 2005b. Geographical distribution of zooxanthella types of three coral species of the Great Barrier Reef sampled after the 2002 bleaching events. Coral Reefs, in press.



0 Comments