In the last 15 years, the ability to recreate a piece of a living reef in ones home has reached unprecedented heights. With the development of high intensity lighting, the maintenance of corals that require light has become possible and is now within the reach of almost any aquarist. Corals such as Acropora, Montipora, and Seriatopora are now commonly grown and reproduced through fragmentation in home aquariums. However, there are still a group of corals that, despite all the advances of the past fifteen years are still proving almost impossible to keep. Although there are isolated cases where some success has been reported, the maintenance of these corals for long periods of time, where growth and propagation have approached natural rates, still remains elusive. Commonly know as azooxanthellate or
non-photosynthetic corals, they include several families and genera. These include soft corals such as Chironephthya, Dendronephthya, Scleronephthya, Siphonogorgia and Stereonephthya, gorgonians such as Acabaria, Acalcygorgia, Melithaea and Subergorgia, black corals and wire corals such as Antipathes and Cirripathes, hydrocorals such as Stylaster and Distichopora, and of course stony corals in the genus Tubastraea. Although the term “soft” coral is used to describe a grouping of corals, it should be noted that this is a generic term used primarily for corals in the suborder Alcyoniia.
The reasons for the poor success rates with the majority of these corals can be traced to fact that they need to feed on plankton. Enough food, of the right type and size must be provided. Until recently, very little was known about the feeding requirements of these corals. The vast majority of soft corals and gorgonians available in the hobby rely greatly on zooxanthellae for their nutrition. However, recent studies have shown zooxanthellae may not be able to meet the total nutritional needs of all soft corals. Fabricius and Klumpp (1995) found that twelve of the most common photosynthetic soft coral species investigated on the Great Barrier Reef could not meet their carbon requirements by photosynthesis alone.
This brings up the question of just where do they get their carbon? Many octocorals are known as polytrophic feeders, meaning that they are capable of obtaining nutrition from more
than one source (Williams, 1993). Possible sources may be one or all of the following: the direct absorption of nutrients, the ingestion of zooplankton and/or phytoplankton, the ingestion of “marine snow” along with its attached bacteria and organic material. Several studies have shown that soft corals, gorgonians and sea pens can feed on a variety of zooplankton such as copepod nauplii and eggs, invertebrate eggs and other small items of poor mobility. Many of these studies, however, were conducted in the laboratory, using artificial foods (Artemia) or concentrated natural zooplankton of unknown density (Fabricius et al., 1995a). These studies showed that octocorals tend to be highly selective for non-evasive forms such as mollusc larvae; indicating poor capture ability of more elusive prey such as large adult copepods. This poor capture ability is most likely due to the lack of effective
nematocysts, resulting in the selection of less motile prey (Fabricius et al., 1995a). In fact, Fabricius (unpublished data) found that an inability to feed on zooplankton was widespread amongst zooxanthellate soft coral genera on the Great Barrier Reef (i.e. three species of Sarcophyton, two species of Sinularia, Cladiella sp., Nephthea sp. and Paralemnalia sp.). The role that zooplankton play in the nutrition of photosynthetic octocorals is, as yet, unclear but new information is showing that they contribute only a small portion to the nutritional budget of many octocorals (Fabricius et al., 1995a and b). However, many of the studies that looked at a corals ability to feed on zooplankton often used Artemia nauplii as prey items under controlled situations. Artemia are rather large, and it may not be surprising given the small size and weak nematocysts of many soft corals, that they are not easily captured. Perhaps smaller zooplankton such as copepod nauplii, rotifers, or marine infusoria is fed upon? However, the question remains, if not zooplankton, then what are their main prey items? Phytoplankton is an order of magnitude more common on coral reefs than zooplankton.
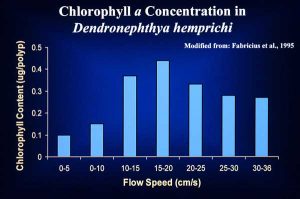
Studies have shown that phytoplankton is somehow depleted over corals reefs, though where it goes no one knows (in Fabricius et al., 1995a). In 1961, Roushdy and Hansen showed that the asymbiotic soft coral Alcyonium digitatum feed on 14C labeled phytoplankton (in Fabricius et al., 1995b). In 1969, it was demonstrated that the temperate water sea pen Ptilosarcusgurneyi fed primarily on phytoplankton; its bright orange colour, the result of carotenoids derived from a diet of dinoflagellates (in Best, 1988). Elyakova et al. (1981), in a general survey of carbohydrases in marine invertebrates, found the presence of laminarinase and amylase in three species of the zooxanthellate soft coral genus Alcyonium, enzymes involved in the digestion of plant material.

It was not until 1995 that Fabricius et al. published papers that demonstrated quite clearly that the Red Sea azooxanthellate soft coral Dendronephthya hemprichi, fed extensively on phytoplankton, gaining more than enough carbon to cover respiration and growth requirements. Although this species also fed on zooplankton, only 2.4-3.5% of the daily carbon requirement of this coral was met by ingesting zooplankton. Three other asymbiotic Red Sea octocorals, D. sinaiensis, Scleronephthya corymbosa and the gorgonian Acabaria, were also found to contain large quantities of phytoplankton in their gastrovascular cavities (Fabricius et al., 1995b). Adaptations for phytoplankton capture include the small spaces between the pinnules of D. hemprichi, which appear to be ideal for straining phytoplankton

from flowing waters. The large spicules found in the body column and around the polyps of Dendronephthya spp., appear to function more in holding the column and polyps erect in strong current flows, than to protect against predation, allowing the polyps to strain phytoplankton effectively from the passing waters (Fabricius et al., 1995a). Some of the most impressive growths of Dendronephthya spp. are often found on shipwrecks in the South Pacific, where structures high above the bottom and projecting into the current are often heavily encrusted. It is tempting to equate this with oyster hatcheries, where oysters are hung in cages well above the bottom and within strong currents. Both organisms feed on phytoplankton, and hence benefit from these positions by being exposed to maximal phytoplankton concentrations. In light of this new evidence, scientists need to re-evaluate the role of phytoplankton in the nutrition of other octocorals. Several studies are now underway to determine to what extent both zooxanthellate and azooxanthellate species actually feed on phytoplankton.

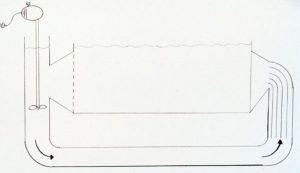
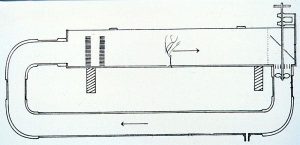
Cylinder tank used for Dendronephthya research. Note central stand pipe and twin return pipes at the back of the tank each connected to a separate pump and timer. Photo: Norton Chan.
Another mode of feeding may be the trapping of mucus flocs often called marine “snow”. These are composed of detritus, bacteria, protozoans and possibly phytoplankton trapped in mucus. The source of this mucus is most likely soft and stony corals, which rid themselves of epizoic growths and excess carbon and fats, by releasing mucus. This mucus is not easily degraded by bacteria and is often infested with large quantities of bacteria and eukaryotes (flagellates, ciliates and diatoms) (Vacelet and Thomassin, 1991). These mucus flocs could be trapped by the spiky polyps of Dendronephthya spp. and used as a food source. It is fully possible that octocorals employ a combination of some or all of the above feeding mechanisms, with varying degrees of importance for each.
Armed with the above information, first presented to hobbyists in The Reef Aquarium volume two, (Sprung and Delbeek, 1997) several North American aquarium supply companies now make available various mixtures of phytoplankton, some live, some cryo-preserved, and some consisting of dead algal cells. Most of these products were originally developed for the aquaculture industry. Although phytoplankton may be of importance to soft corals, its direct role for other non-photosynthetic corals is not as well demonstrated, and may indeed be questionable. Certainly corals such as Tubstraea spp. and antipatharians are known to prey heavily on zooplankton. The needs of some gorgonians can also be well-met using zooplankton substitutes such as enriched Artemia, rotifers and copepods.

Tank soon after the addition of corals. Photo: Norton Chan
As it turns out, the foods required for success are only one piece of the puzzle. Another factor, equally important is water motion; not only the type but also the velocity of water motion can be critical for some genera, but less critical for others. Given that octocoral polyps have few, small stinging cells (or none at all) and that their pinnules offer a large surface area, they are generally classified as suspension feeders, straining fine particles from the passing water. As such their feeding efficiency is affected by the rate of current flow, polyp and colony flexibility, and orientation. Several studies have shown that feeding efficiency generally increases up to a maximum velocity and then drops off at velocities beyond that (Best, 1988; Sponaugle and LaBarbera, 1991; Dai and Lin, 1993; Fabricius, et al., 1995a). However, the flexion of the polyps and colony can act together to increase the range of current velocities over which suspension feeding is successful (Sponaugle, 1991). The polyps themselves can actually modulate the flow around them, to enhance prey capture. In a study of the effects of flow on particle capture in the asymbiotic temperate octocoral Alcyonium siderium, Patterson (1991) found that at low flows (2.7 cm/s) tentacles on the upstream side of the polyps capture the most prey. At intermediate flows (12.2 cm/s) downstream tentacles within a polyp capture the most prey. In high flow (19.8 cm/s) polyps are bent downstream, eddies form over the polyp surfaces and all tentacles capture prey effectively. Prey is trapped most effectively at the tips of the tentacles relative to locations near the mouth (Patterson, 1991).

Tank soon after filling. Photo: Norton Chan
No one current flow is the best for all species. For example, Dai and Lin (1993) found three Taiwanese asymbiotic gorgonians Subergorgia suberosa, Acanthogorgia vegae and Melithaea ochracea to feed over a wide range of flow rates. The ability to keep polyps open was also related to flow rates and the size of their polyps. Subergorgia suberosa had the largest polyps, which were deformed by the lowest currents speeds (>10 cm/s), severely hindering prey capture. In contrast, Melithaea ochracea, which had the shortest and the least easily deformed polyps at high flow rates, could feed at the highest flow rates (40 cm/s). Acanthogorgia vegae had an intermediate polyp size and fed in flows of 0-24 cm/s. Although all three fed most effectively at flows of 8 cm/s, S. suberosa had the narrowest feeding range (5-10 cm/s) while M. ochracea had the widest range (4-40 cm/s) (Dai and Lin, 1993). This varying ability to feed in various current flows is a major factor in determining distribution on reefs. Melithaea ochracea is the most widely spread gorgonian on southern Taiwanese reefs, occurring on the upper part of reef fronts where currents are strong. Subergorgia suberosa, which feeds in a narrow range of flow velocities, has a restricted distribution pattern, being found on lower reef slopes or on sheltered boulders where currents are weaker. Acanthogorgia vegae, which can feed in relatively strong currents, is most commonly found on the semi-exposed reef fronts or the lateral side of boulders (Dai and Lin, 1993).
Therefore, water flow and its interactions with polyps and colonies, appears to greatly influence distribution patterns of colonies, colony growth, size and morphology, and rates of gas exchange (in Fabricus et al., 1995a). To summarize, in increasing flows, feeding rates initially increase, peak, then decrease as flow rate increases. Having too great a flow can also cause polyps to stay open for shorter and shorter periods of time and having flow rates too low do not stimulate polyps to open and feed.
In aquaria, water motion is predominantly of two types, laminar and chaotic. Laminar flows occur close to the outlets of powerheads and water returns. Chaotic flows begin to appear further from these sources as the flowing water encounters resistance from water, tank walls, rocks and corals. Areas where two water flows intersect also provide areas of chaotic water
motion. However, the areas where the majority of non-photosynthetic corals appear on reefs, (along drop-offs and channels in reefs) have laminar flows that usually operate on tidal cycles. Water flow will gradually increase in one direction then decrease, then change direction and increase again. There are periods of “slack” tides between these two extremes. The corals in these regions therefore receive strong laminar flow in one direction for several hours followed by a slack period, then another period of strong water flow in the opposite direction for several hours. To stimulate this in aquaria is difficult and may require the design of new tank designs and water motion devices.

Dendronephthya colonies located along outer edge where greatest flow rate was attained. Photo: Norton Chan.
At the Waikiki Aquarium we have been working with a cylindrical tank to simulate these conditions with two waterpumps operated by timers. The tank contains live sand, some live
rock and has a continuous flow of natural seawater, and so no other filtration is required. Lighting is supplied by ambient natural sunlight coming from overhead acrylic panels with some direct sunlight for a few hours each day. Other tank designs such as flumes and flow tanks could also be used. For example, at the Vancouver Aquarium, in Canada, an exhibit of coldwater gorgonians has been constructed that uses a flow tank with high horsepower pumps to simulate the very strong tidal currents that occur in some of the passes between the islands offshore of British Columbia.
At the Waikiki Aquarium we have had some moderate success with keeping certain species of black coral, and good success with wire corals by feeding them a diet of enriched Artemia and copepods. The Long Beach Aquarium of the Pacific in California has had some success with Dendronephthya, Distichopora and some other soft corals using a diet of phytoplankton including Chlorella sp., Spirulina sp., Isochrysis sp., and Nanochloropsis sp. To that algae “soup”, they add rotifers and supplemented Artemia. They also found that Distichopora, unlike non-photosynthetic soft corals, unquestionably require low light levels. If left in even moderate light, fouling organisms quickly adhere to their delicate tissues and result in mortality. The Shedd Aquarium in Chicago, has had one colony of Dendronephthya for over a year, which has shown noticeable growth. They are feeding phytoplankton as well as live rotifers and copepods. Our success with Dendronephthya has been less inspiring.

Dendronephthya along outer edge. Photo: Norton Chan.
In early December 2000, we collected fifteen small colonies of Dendronephthya in Fiji and transported them back to Hawaii under permit. At present (March 17, 2001) we have seven of the fifteen colonies still surviving. Although we have tried several food types such as live marine phytoplankton (Chaetoceros, Isochrysis, Nannochloropsis etc.), copepods, rotifers, fatty acid supplements and “marine snow” products, we have had mixed results. In some cases, damaged colonies quickly regrow polyps and attain new tissue, however, established colonies slowly decreased in size. Interestingly enough, the greatest reaction to substances added to the aquarium occurs in two ways. When the interior of the glass is wiped of algae the colonies show an increase in size only an hour or so later. Secondly, when the juice from thawed frozen squid is added to the aquarium, colonies show the greatest increase in expansion. It is likely that these corals feed to a greater extent on zooplankton then current research has indicated and aquarists should not rely solely on phytoplankton as a food source. Peter Wilkens has been able to keep small colonies for some time in his aquaria by occasionally stirring the bottom substratum, releasing detritus and quite possibly bacteria and other infauna, on which the corals may feed.

Dendronephthya sp. at 120 ft., Solomon Islands. Photo: JC Delbeek.
In April of 2001, our director Dr. Bruce Carlson returned from Fiji with a specimen of the gorgonian Menella. With its magenta tissue and snow-white polyps it is a beautiful sight when fully opened! As with the Dendronephthya we had collected previously, this coral opens its polyps most frequently when squid juice is added or the tank window is cleaned. While closely observing the polyps, live Artemia naupilli, live rotifers and copepods were added in separate feeding trials. Even polyps that had Artemia directly applied to them could not hold onto the struggling live food, and were eventually released. Next, live microalgae cultures were tried as well as Algamac, a commercially available artificial phytoplankton substance (see www.argent-labs.com). During these trials individual arms of the polyps could be seen periodically bending towards the mouth, wiping along the aboral surface of the polyp. I believe that mucus on these arms and their associated pinnules may be trapping passing phytoplankton cells then passing them to the mouth to be ingested.

Dendronephthya sp. Sulawesi, Indonesia. Note large red spicules embedded in the tissue for added support. Photo: JC Delbeek
Orientation of colonies is another factor that can play a significant role. Colonies that were placed upright on the sand bottom in our system initially appeared to do well, but over time, began to shrink in size. When placed upside-down from a supporting structure, these colonies slowly recovered and looked much better, some even showing new growth and reattachment to the substratum. The key seems to be to not allow the colonies to touch the bottom with their branches; this seems to irritate them over time and results in the loss of spicules. This is of greatest concern when the colony is deflated. Another interesting observation is that colony inflation and deflation does not seem to follow any discernable pattern. In the early morning the colonies are deflated and then inflate later in the morning and would remain so for most of the day, although deflation could occur again at any time during the day.
As I mentioned briefly above, the Waikiki Aquarium has had some success in maintaining live wire corals and black corals. The wire coral Cirrhipathes anguina was easily maintained on live Artemia naupilli enriched with Super Selco and Algamac, and growth was readily visible. The black coral, Antipathes dichotoma, fed on live copepods but growth was not as noticeable and the colonies would slowly deteriorate with time. Recently, I tried feeding this coral frozen copepods from Argent called Cyclopeez, which I was feeding to my Pseudanthias tank at the time. These were rather large compared to Artemia, about twice as wide. They are also enriched with various pigments and appear bright orange/red. I placed a small branch in a dish of seawater and placed this under a dissecting microscope. I used an eyedropper to add a small amount of Cyclop-eez to the expanded polyps. To my surprise the polyps easily grasped these large copepods and would engulf the entire animal by expanding the mouth until the entire animal could be passed in. Apparently the fact that these were not moving targets helped the polyps capture and ingest them as opposed to a struggling live prey item. I assume that the lack of any water movement also made prey capture easier.

Scleronephthya with its distinctive dark polyp mouth and light tentacles, has proven easier to keep than Dendronephthya spp. Photo: JC Delbeek.
Unfortunately, I have not had time to pursue this due to other projects, but I would like to attempt a long term study by feeding this food item to a colony over a period of months and see whether or not it’s growth and survival improves over that of copepod and Artemia fed colonies.
There are several questions that remain to be answered in keeping non-photosynthetic corals. The role of temperature, colony orientation, nutritional composition of foods (including pigments), food density, the best techniques for coral collection, and handling and shipping are just some that need to be investigated over the next few years. We are beginning to see limited success with many of the non-photosynthetic corals that used to be very difficult to keep, and it is only a matter of time until tanks filled with colorful, healthy non-photosynthetic organisms will be as common as tanks filled with Sarcophyton are today.
Bibliography
- Best, B.A. 1988. Passive suspension feeding in a sea pen: effects of ambient flow on volume flow rate and filtering efficiency. Biol. Bull. 175:332-342.
- Dai, C.D. and M.C. Lin. 1993. The effects of flow on feeding of three gorgonians from southern Taiwan. J. Exp. Mar. Biol. Ecol. 173:57-69.
- Elyakova, L.A., Shevchenko, N.M. and S.M. Avaeva. 1981. A comparative study of carbohydrase activities in marine invertebrates. Comp. Biochem. Physiol. 69b:905-908.
- Fabricius, K.E. and D.W. Klumpp. 1995. Wide-spread mixotrophy in reef-inhabiting soft corals: The influence of depth and colony expansion and contraction on photosynthesis. Mar. Ecol. Progr. Ser. 126:145-152.
- Fabricus, K.E., Genin, A. and Y. Benayahu. 1995a. Flow-dependant herbivory and growth in zooxanthellae-free soft corals. Limnol. Oceanogr. 40:1290-1301.
- Fabricus. K.E., Benayahu, Y. and A. Genin. 1995b. Herbivory in asymbiotic soft corals. Science 268:90-92.
- Patterson, M.R. 1991. The effects of low on polyp-level prey capture in an octocoral, Alcyonium siderium. Biol. Bull. 180:93-102.
- Sponaugle, S. 1991. Flow patterns and velocities around a suspension-feeding gorgonian polyp: Evidence for physical models. J. Exp. Mar. Biol. Ecol. 148:135-145.
- Sponaugle, S. and M. LaBarbera. 1991. Drag-induced deformation: a functional feeding strategy in two species of gorgonians. J. Exp. Mar. Biol. Ecol. 148:121-134.
- Sprung, J. and J.C. Delbeek. 1997. The Reef Aquarium: A Comprehensive Guide to the Identification and Care of Tropical Marine Invertebrates. Ricordea Publ., Coconut Grove, FL, USA, 546 pp.
- Vacelet, E. and B.A. Thomassin. 1991. Microbial utilization of coral mucus in long-term in situ incubation over a coral reef. Hydrobiologia 211:19-32.
- Williams, G.C. 1993. Coral Reef Octocorals: An Illustrated Guide to the Soft Corals, Sea Fans and Sea Pens Inhabiting the Coral Reefs of Northern Natal. Durban Natural Science Museum, Durban, South Africa. 64 pp.





0 Comments