
While a great deal of interest has been shown in the characteristics of artificial daylight for reef aquaria, very little attention has been paid to the other natural illumination – moonlight. Although manufacturers have marketed moonlight simulators for a number of years, I’ve yet to see an in-depth discussion of the subject. This article will attempt to address that issue while discussing some misconceptions about lunar light. In addition, we’ll define spectral characteristics of moonlight, light intensity, and length of natural lunar photoperiod, and ways to simulate moonlight. We’ll also examine the effects (or non-effects) of moonlight on timing of coral spawning (and comment, albeit briefly, its effects on fish spawning behavior).
Lunar Photoperiod in Hawai’i
As we know, the lunar cycle consists of 29.5 days and is the basis for our calendar month. The lunar phase changes in a predictable manner and is due to relative positions of the moon, earth, and sun. Phase is not due to the earth’s shadow falling upon the moon (this is referred to as a lunar eclipse). Figure 1 shows phases and approximate and approximate days of the lunar month.
Figure 2 shows the hours of potential moonlight in Hawaii. Data are based on times of sunrise/sunset and moonrise/moonset.
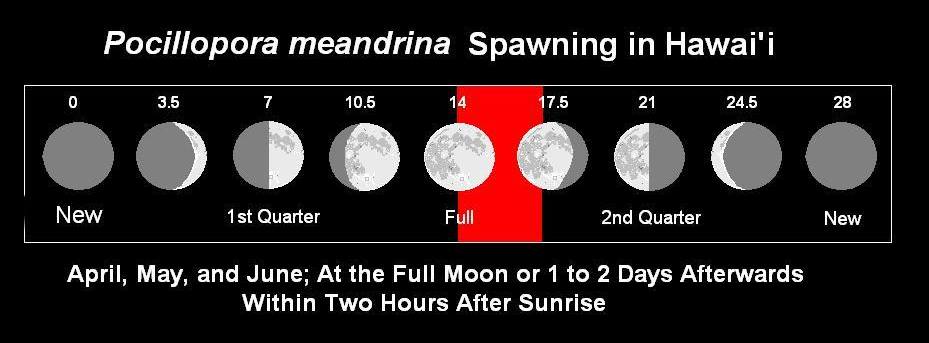
Figure 1. The lunar cycle along with comments on the spawning activity of stony corals Pocillopora meandrina (as well as P. eydouxi) in Hawaii. The numbers above the moon phases indicates is the approximate time of the cycle in days. The red bar is the window for potential coral reproduction during the spawning season.
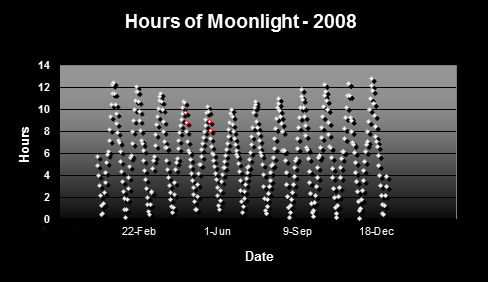
Figure 2. Hours of moonlight in Hawai’i (latitude N 1938′). Red dots indicate major spawning events of Pocillopora meandrina and Pocillopora eydouxi in waters off the west side of the Big Island of Hawaii.
Moonlight Spectral Characteristics
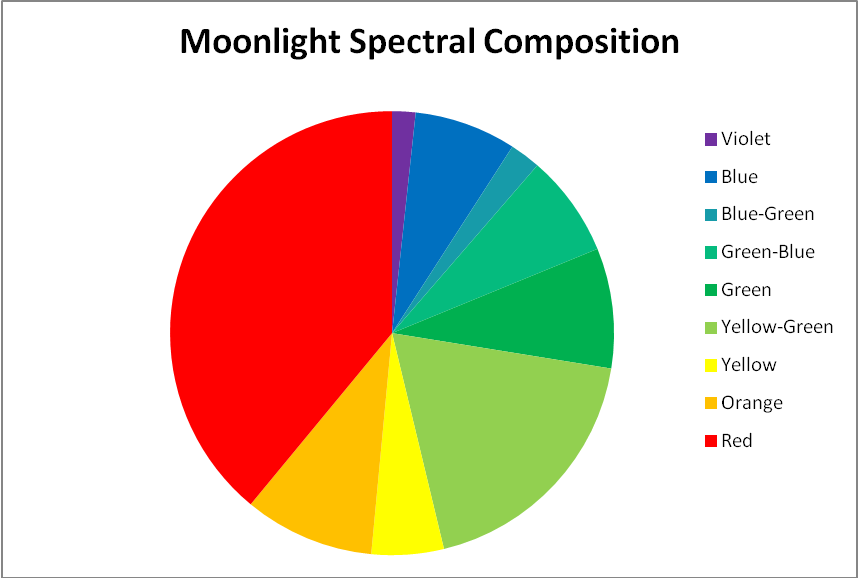
Since moonlight is almost entirely reflected sunlight, one might reason that the moon’s spectral signature is exactly that of sunlight – it is not. Data shown in Figures 3 & 4 reveal that moonlight is less blue and redder than sunlight (and this measurement was taken with a ‘silvery’ moon at its zenith. We often see a much more orange moon at moonset).
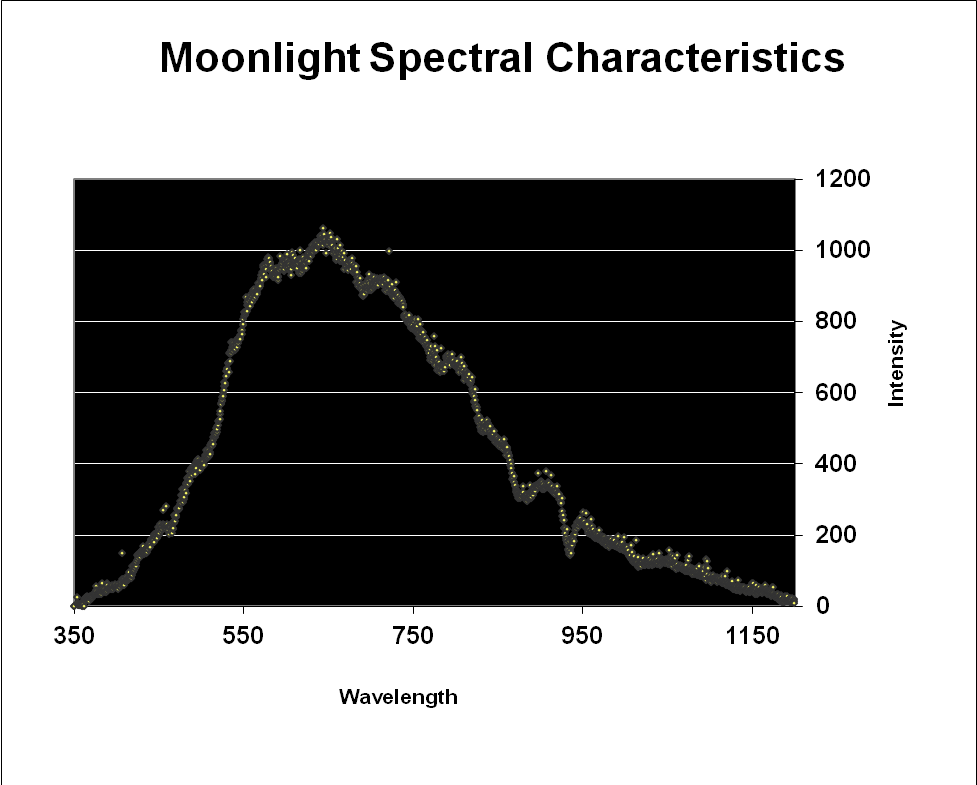
Figure 3. Moonlight peaks in the red portion of the spectrum (643nm) but appears ‘silvery’ when at its zenith on a clear night.
Moonlight Intensity
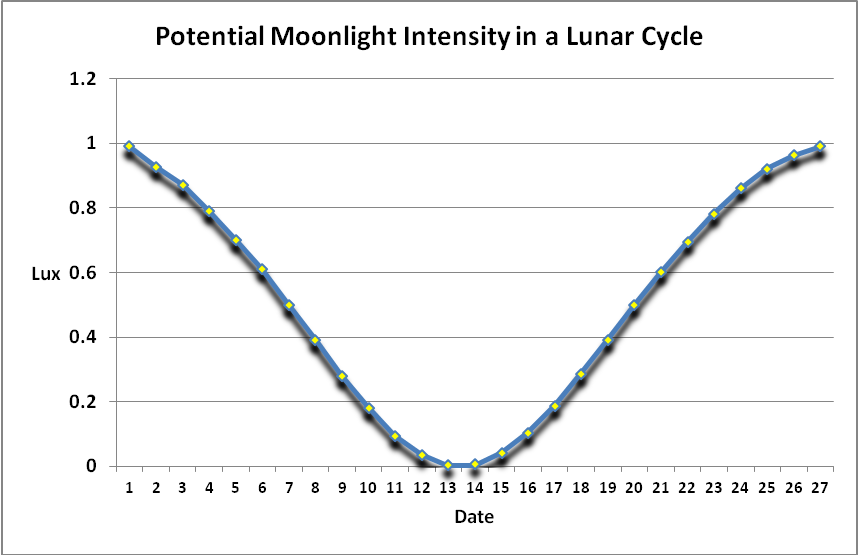
Moonlight intensity is determined by lunar phase and sky conditions. Figure 5 shows moonlight intensity (in lux) under ideal conditions. Figures 6 and 7 show full moon light intensities (PAR) as measured during two nights (just a few feet above sea level). Note that the intensities are lower than that reported by Jokiel (0.05 µmol·m²·sec, or about 1 lux). The low moonlight intensity reported here is due to a number of factors, including seawater aerosols in the air, thin high level clouds, and vog (a mixture of atmospheric moisture and volcanic smoke from the Pu’u O’o vent and Halema’uma’u caldera of the Kilauea volcano).
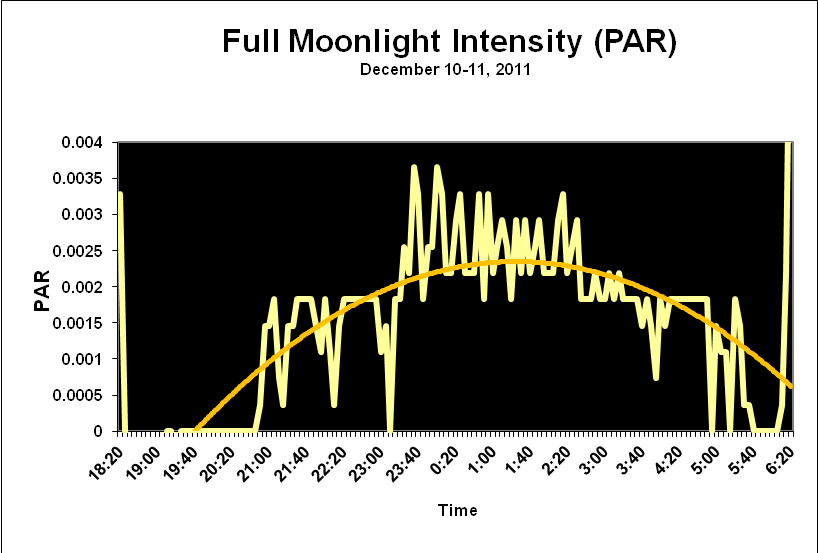
Figure 6. Actual light intensity of a December full moon in Kailua-Kona, Hawaii as recorded by a PAR data logger. Thin, high level caused the moon to have a halo and reduced intensity.
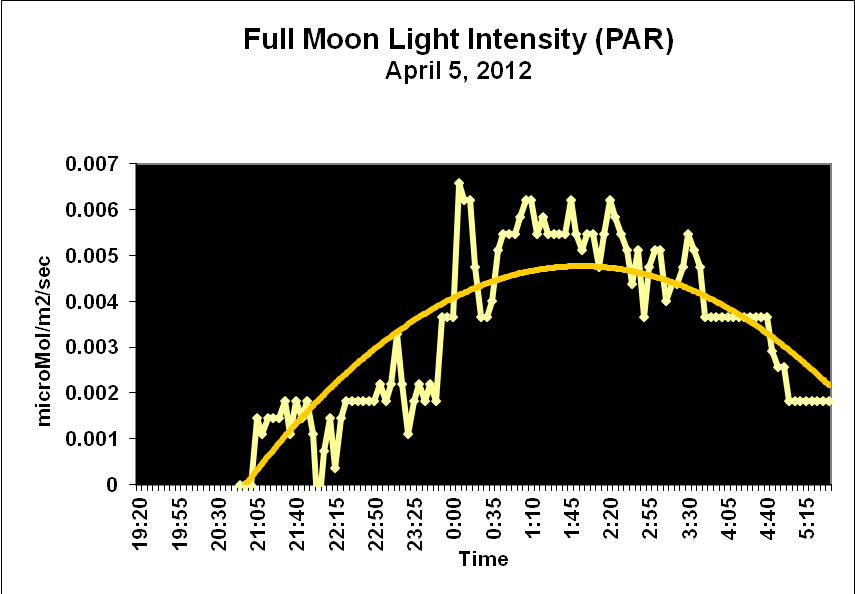
Figure 7. Actual light intensity of a full moon two days before a seasonal spawning of Pocillopora meandrina and P. eydouxi stony corals in Kailua-Kona, Hawaii.
Factors Influencing Coral Reproduction – Order of Importance
Moonlight is but one factor influencing coral reproduction. If other factors (nutrition, physical parameters, etc.) are correct, these are believed to be important:
Temperature: Temperature seems to exert powerful control over coral reproduction. If the temperature is too high, coral health can suffer, while cool temperature may delay spawning until the next month’s window (Hunter, 1988; Riddle personal observations). Temperature has been stated to be the influence of paramount importance in the reproductive cycles of marine invertebrates (Olive, 1995). In Hawaii, the temperature threshold is about 75F (24C; Dr. Paul Jokiel, personal communication).
Moonlight: Lunar cycles set the date of spawning in many coral species and the lunar calendar can be used to accurately predict it.
Daylight Photoperiod: Solar photoperiods influence coral reproductive efforts and set the hour and minute of spawning (Vize et al., 2008). The time of sunset is the fine-tuning factor for many marine invertebrates including at least some sponge and coral species.
Corals Don’t Have Eyes – How Do They Sense Light? And What Do They See?
Gorbunov et al. (2002) found blue light at about 480nm (110nm width, half maximum) at very low light intensity caused a reaction among coral tentacles,although a description of photoreceptors involved was not part of the experiment.
In 2003, Levy et al. exposed corals (azooxanthellate Cladopsammia gracilis) the bubble coral Plerogyra sinuosa, the flower pot coral Goniopora lobata, Favia favus, and Stylophora pistillata) to various light wavelengths (400-700nm at 20nm intervals) and intensities (10µmol·m²·sec and 30 µmol·m²·sec; ~500 lux and 1,500 lux, respectively) and recorded tentacle contractions. Cladopsammia did not respond to any light treatment, while Plerogyra sinuosa and Favia favus contracted their tentacles when exposed to wavelengths between 400-520nm (violet-blue-green). Interestingly, Favia favus also responded to red light (660-700nm) at 30 µmol·m²·sec or ~1,500 lux (see light sensitivities of rhodopsin-like compounds and cryptochromes below).
Five years later, a rhodopsin*-like compound was found in the stony coral Acropora millepora (Anctil et al., 2007), explaining how corals sense light. Almost simultaneously, Levy et al. (2007) described cryptochrome** proteins sensitive to blue light in Acropora millepora. Other researchers have noted corals’ responses to light suggesting rhodopsin-like compounds are found in at least some corals.
This ability to sense light explains how corals can grow towards light, and if overturned, can redirect their growth (this is call phototropism). It also explains how corals set their biological clocks through sensing daylight and moonlight.
*Rhodopsin is a photosensitive pigment found in many animals’ eyes (including humans) within receptors called cones. Cones and their rhodopsin content enable us to see in very low light conditions. Rhodopsin collects light in wavelengths of about 400nm (violet) to red (at ~600nmn) but most strongly in the blue-green portion of the spectrum (Hunt, 1987).
**Cryptochromes (Greek for ‘hidden color’) are proteins sensitive to blue light and are found in photoreceptors of plants and animals.
Entrained Biological Rhythms versus Response to Environmental Factors
The act of coral spawning involves production of a number of compounds, and this may be the result of entrained rhythms or exposure to external stimuli. For our purposes, entrained rhythms are those that occur without external stimuli such as sunlight or moonlight. These are likely controlled genetically. Environmental factors (such as like or moonlight) can influence the production of compounds. Vize et al. (2008) found photoreceptors signal production of proteins important in annual spawning of the stony coral Montastrea cavernosa.
Fish Reproduction and Lunar Phase
Many fishes are known to spawn synchronously around a certain lunar phase and this timing may be species-specific. For instance, Takemura et al., 2004 discuss lunar phase and spawning of the golden rabbitfish (Siganus guttatus). These fish did not spawn when subjected to constant illumination, and those held in conditions of total darkness at night displayed altered spawning patterns. Pressley (1980) described the relationship of lunar phase and reproduction of the yellowtail damselfish, Microspathodon chrysurus.
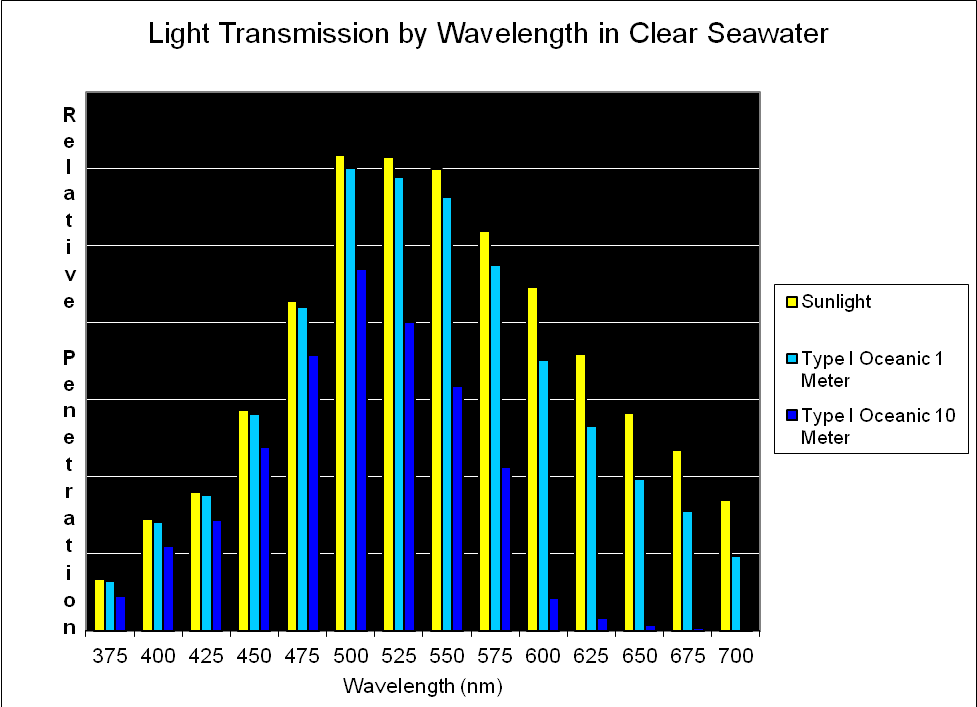
Figure 8. Transmission of light (by wavelength at 25nm intervals) through the clearest of seawater (Type I Oceanic; after Jerlov, 1976). Note that blue-green light at ~500nm penetrates this water the best.
It is an interesting notion that circadian rhythms play an important part in fish reproduction and that accurate simulation of lunar phase may be an important factor.
Light Spectra Transmission in Clear Seawater
As mentioned earlier, several researchers have found that some corals respond to blue light. It is perhaps not by coincidence that maximum penetration of light occurs at about 480-500nm. See Figure 8.
Moonlight and Coral Spawning
Moonlight is commonly believed to be one of the deciding environmental factors for timing of coral spawning. Jokiel (1985) examined numerous Pocillopora damicornis specimens and concluded planula release occurred around the time of the full moon. However, Hunter (1988) experimented with two Hawaiian Montipora species (M. verrucosa = capitata and M. dilatata) and found the following:
- Both sets of corals spawned simultaneously with control corals when exposed to constant simulated moonlight (at a flux of 0.01 µmol·m²·sec, or about 0.5 lux)
- When exposed to no simulated moonlight (constant new moon), 43% of the M. verrucosa spawned in sync with the controls, and in the next month, 1 week prior to the new moon. Montipora dilatata specimens also spawned in synch with controls in the first month, and then 8 days out of normal phase the next month.
- When maintained under simulated moonlight shifted 14 days out of phase, both coral species spawned simultaneously with controls, and then 2 to 12 days out of sync in the second month.
Artificial Moonlight
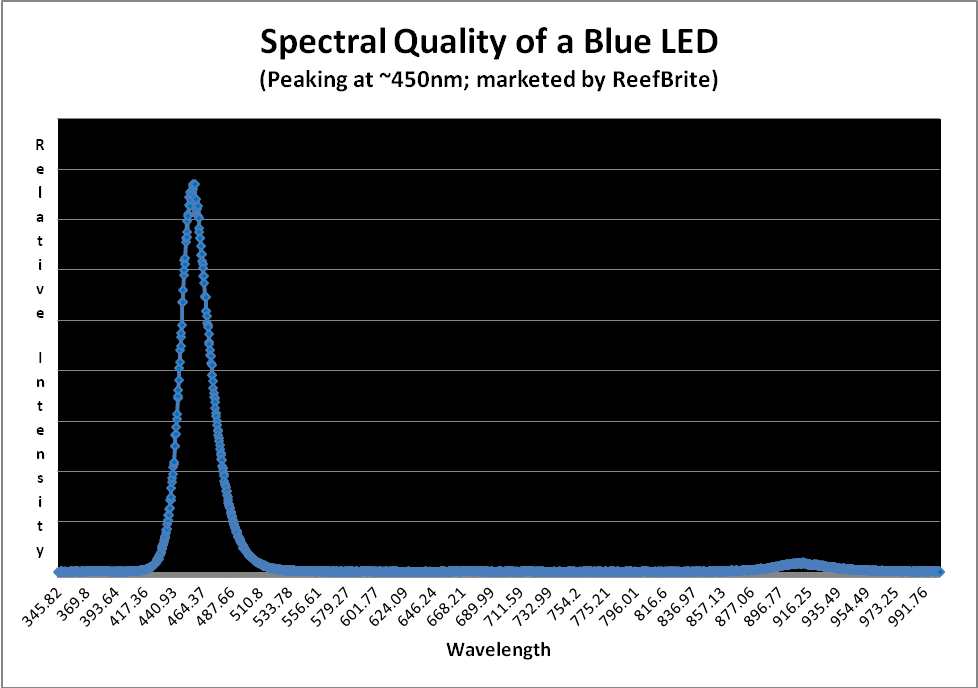
It is usually impractical to expose an aquarium to moonlight hence artificial means are preferred. In my 1995 book, The Captive Reef, I outlined a means of simulating moonlight with a blue incandescent lamp and a manual dimmer. Technology has come a long way since then and light-emitting diodes are now the preferred method. See Figure 9.
Figure 10 shows the typical spectral quality of a LED peaking in the blue portion of the spectrum at ~450nm.
Controllers
There are a number of controllers on the market claiming to simulate timing and variable intensity of natural moonlight. This article is not intended to review all those available. Instead, I describe the one I own – the tunze Multicontroller 7095. This device’s main function is that of controlling tunze pumps but includes a LED for moonlight simulation. The only thing a hobbyist has to do is turn the moonlight LED on when the real moon is full and the controller automatically does the rest. A photo-sensor will turn the LED moon on when the aquarium lights go out and lunar phase intensity is controlled over a 29 day cycle. See Figure 11 for a close up view of the photo-sensor/LED and Figure 12 shows the spectral characteristics of the LED.
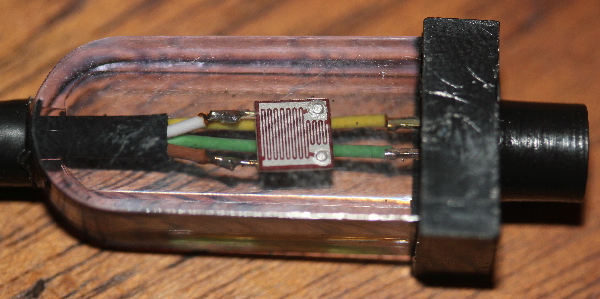
Figure 11. The photosensor of the Tunze 7095 Multicontroller is housed in clear acrylic. When the lights go out, this sensor automatically turns the LED on (in the black tube to the right) and vice versa. This assembly is less than 2 inches (5cm) long.
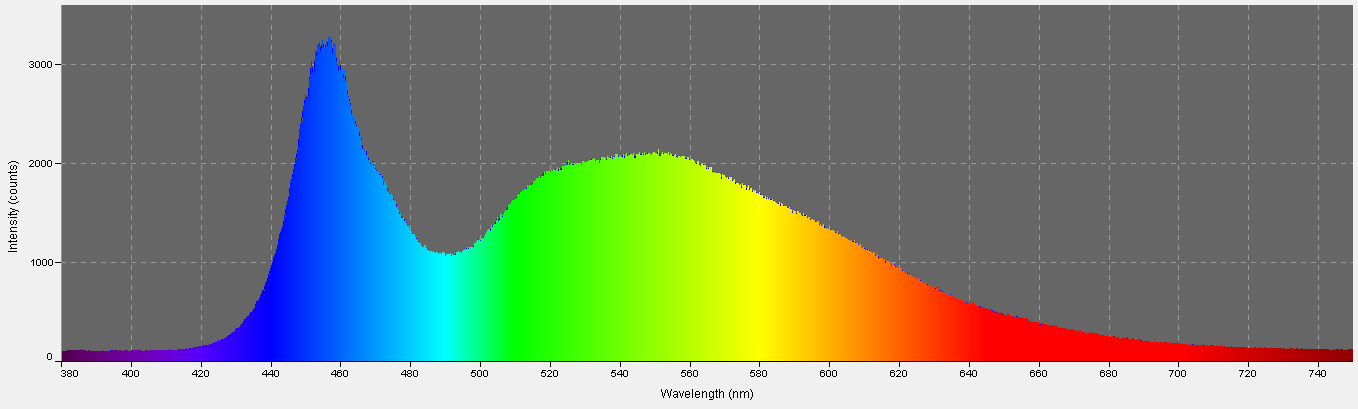
Figure 12. Spectral quality of the Tunze LED moon. It is full-spectrum, with peak intensity at about 460nm.
In Closing
Many corals contain photoreceptors (note their ability to almost always grow towards light). Some demonstrate responses to blue light, while at least one species can sense both blue and red light. Some show no response to light.
Moonlight is thought to play an important role in timing reproductive cycles of many coral and fish species. In corals, lunar cycles set the date of spawning, while the time of onset of darkness fine tunes the cycle and decide the hour and minute (then a release of hormones into the water induces mass spawning). An altered lunar phase may at least temporary disrupt spawning synchrony among at least some coral species. Lunar periodicity seems to play a role in timing of reproduction among at least some fish species. Interestingly, short term exposure of some fishes to constant artificial moonlight may have prevented spawning, while the same did not affect the patterns in some corals. It seems apparent that different taxa are affected differently by altered moon phases, if only temporarily.
Although moonlight appears white or silvery, use of LEDs producing blue light to simulate moonlight is, at least for some coral species, correct based to peer-reviewed evidence. Use of LEDs producing white light is likely to be OK as well, since these diodes are essentially blue LEDs doped with phosphors that fluoresce longer wavelengths. However, the light intensity of the light produced by even a single blue LED has the potential to be brighter than natural moonlight measured here in Hawaii. Light penetration in aquaria, with their usually shallow (and hopefully clear!) waters, should not be an issue, so using LEDs with a maximum wavelength of 450 or 460nm may actually be an advantage due to their lower output at 480nm.
Since most PAR meters’ minimum respond is ‘1’, these units are useless in determining proper placement of a light source in order to mimic natural moonlight intensity. On the other hand, a lux meter can measure moonlight at its maximum intensity although the reading will be ~1. Hence, placement of the LED for providing proper intensity will likely have to be estimated visually. At present, the effects of over-illumination of a reef aquarium at night are unknown but it is possible that it might affect fish or invertebrate spawning behavior.
A number of controllers with abilities to simulate lunar phase are on the market. In absence of one, a handy hobbyist can make a manually-controller lunar simulator with a low wattage incandescent lamp and a rheostat.
Testing Equipment
Spectral characteristics of the moon and LED were measured with an Ocean Optics USB2000 spectrometer and SpectraSuite software. Data were downloaded to an Excel worksheet for post-processing. Moon intensities were recorded by a Li-Cor 1400 quantum meter/datalogger and cosine-corrected quantum sensor.
Acknowledgement
Thanks to my brother David for supplying the photograph of the moon.
Questions? Comments? Please post below or contact me at [email protected].
References
- Anctil, M., D. Hayward, D. Miller, and E. Ball, 2007. Sequence and expression of four coral G protein-coupled receptors distinct from all classifiable members of the rhodopsin family. Gene, 392(12): 14-21.
- Brady, A., K. Snyder and P. Vize, 2011. Circadian cycles of gene expression in the coral, Acropora millepora. PLoSOne Online.
- Gorbunov, M., Z. Kolber, M. Lesser, and P. Falkowski, 2002. Photoreceptors in the cnidarian hosts allow symbiotic corals to sense blue moonlight. Limnol. Oceanogr., 47(1), 2002, 309-315.
- Hunt, R., 1987. Measuring Colour. Fountain Press, Kingston-upon-Thames, England. 344 pp.
- Hunter, C., 1988. Environmental cues controlling spawning in two Hawaiian corals Montipora verrucosa and M. dilatata. Proc. 6th Int. Coral Reef Symp., Australia. 2:727-732.
- Jerlov, N., 1976. Marine Optics. Elsevier Oceanography Series, Elsevier Sci. Publ. Co., New York. 231 pp.
- Jokiel, P., 1985. Lunar periodicity of planula release in the reef coral Pocillopora damicornis in relation to various environmental factors. Proc. 5th Int. Coral Reef Congress, Tahiti. 4: 307-312.
- Levy, O., L. Appelbaum, W. Leggat, Y. Gothlif, D. Hayward, D. Miller, O. Hoegh-Guldberg, 2007. Light-responsive cryptochromes from a simple multicellular animal, the coral Acropora millepora. Science, 318 (5849):467-470.
- Levy, O., Z. Dubinsky, and Y. Achituv, 2003. Photobehavior of stony corals: Responses to light spectra and intensity. J. Exp. Biol., 206: 4041-4049.
- Olive, P., 1995. Annual breeding cycles in marine invertebrates and environmental temperature: Probing the proximate and ultimate causes of reproductive synchrony. J. Therm. Biol., 20(1, 2): 79-90.
- Pressley, P., 1980. Lunar periodicity of the yellowtail damselfish, Microspathodon chrysurus. Environ. Biol. Fishes, 5:155-159.
- akemura, A., E. Susilo, M. Rahman and M. Morita, 2004. Perception and possible utilization of moonlight intensity for reproductive activities in a lunar-synchronized spawner, the golden rabbitfish. J. Exp. Zoology, Part A: Comp. Exp. Biol., 301A, 10: 844-851.
- Vize, P., J. Hilton, A. Brady and S. Davies, 2008. Light sensing and the coordination of coral broadcast spawning behavior. Proc. 11th Int. Coral Reef Symp., Ft. Lauderdale, Florida.




can I just say amazing work!
Really enjoyed this. As a hobbyist studying to be a marine biologist, it was nice to find a study identifying the effects of moonlight, especially how it may affect an aquarium. A lot of hobbyists think it’s pointless but I feel it might be essential in adequate “ocean” simulation.