Some of the information within this article was presented at the Marine Aquarium Conference of North America (MACNA) in Pittsburgh. Since it is difficult at best to present even the highlights of a year’s research in a one-hour presentation, I appreciate the opportunity offered by the Advanced Aquarist staff for allowing me to ‘flesh out’ that presentation with many ‘new’ details in a written format.

Figure 1. The purple portion of this Acropora specimen is merely reflecting light falling upon it and is not fluorescent.
A Review
All the information presented in the previous six installments of this series has been only a precursor to this article, where we identified coral pigments as described in scientific literature. In this and following articles, we will begin to apply what we’ve learned and make some sense of it.
Major Groups of Colorants
For our purposes, we can divide coral pigments into several different categories:
- Fluorescent proteins. These proteins absorb light energy at a certain wavelength and emit (fluoresce) it at another lower energy wavelength. These pigments ‘glow’ when viewed with actinic and ‘backlight’ lamps. There are about 85 identified fluorescent proteins, but there are likely thousands.
- Photosynthetic pigments. Chlorophyll, phycoerythrin, and marienne. Zooxanthellae are the most common photo-symbionts found within corals, and these dinoflagellates of course contain photopigments including chlorophyll a (which fluoresces a deep red color but is seen only under rather special circumstances). Phycoerythrin is found in cyanobacteria that can also infect coral tissues. Phycoerythrin fluoresces a bright orange color. This orange pigment is visible under conditions less demanding than chlorophyll. ‘Marienne’ is a non-fluorescent but highly reflective pigment found in algae infesting tissues of clams and corals.
- “Kindling” Chromoproteins. “Kindling” is a term that refers to various proteins’ abilities to change from a non-fluorescent chromoprotein to one that fluoresces.
- Non-fluorescent ‘reflective’ proteins – Chromoproteins. These pigments are produced by the coral animal and can appear red, purple, blue, mauve or other colors. There are only a dozen or so described chromoproteins but there are likely many more. These proteins appear rather dull (usually a drab bluish color) when illuminated by high kelvin lamps, but may appear reddish when the light source has a ‘warm’ (lower) kelvin rating. There are only two dozen or so chromoproteins found in corals.
In this article, we will examine how chromoproteins are identified and discuss other pertinent information.
The previous six articles in this series have examined fluorescent pigments (including photopigments) as well as non-fluorescent chromoproteins. In general, fluorescent pigments are produced by the coral host (but see exceptions below). There are at least 85 described fluorescent pigments, each with different peak emission wavelength. If we consider the molecular structure of these pigments, there are probably hundreds, if not thousands. Non-fluorescent chromoproteins have received less attention from researchers – there are only a couple of dozen described absorption peaks. Again, further investigations at finer scales will likely reveal many more non-fluorescent pigments.
As we have seen, pigments are often found in combination with other colorants (Figure 2 shows an excitation and emission spectra of 5 fluorescent pigments within a single coral colony). Some corals will contain more, perhaps a dozen or more fluorescent pigments.
Some fluorescent or reflective pigments are associated with photosynthetic symbionts. Chlorophyll (most often found in zooxanthellae, for our purposes anyway) can, under certain conditions, lend a deep red fluorescent coloration. Another photopigment, phycoerythrin, can, in some cases, make the animal host appear fluoresce orange. Marienne is a reflective pigment found in cyanobacteria and can make clams or corals appear blue-green (Note that marienne is not known to occur in Tridacna clams. Tridacna colors are known to be due to the presence of refractive tissue layers). Any of these pigments have the potential, either through fluorescence or reflectance, to modify the apparent coloration of the host animal.
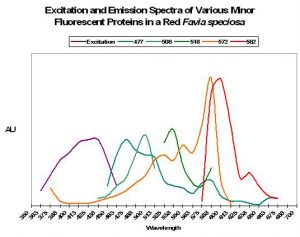
Figure 2. Some corals contain up to 12 fluorescent proteins – this Favia only has five! After Salih et al., 2006.
As reef hobbyists, most of us are probably interested in what it takes to make corals colorful, and once this coloration is expressed, how to maintain or, in certain cases, how to manipulate coloration for our viewing pleasure.
There is now little doubt that light, especially violet and blue wavelengths (and to a lesser degree, ultraviolet ‘light’), are important in rearranging molecular structures of chromoproteins and fluorescent proteins, thus producing the vivid colorations that are so desirable in display aquaria. For many years, there was anecdotal evidence within the hobby that spectra of high-kelvin lamps (10,000-20,000K) were responsible for apparent coral shifts in many captive corals (I personally believe this is partially true. Many of the fluorescent colors are visually striking when the viewing lamp emits a high proportion of excitation wavelengths -violet and blue – while also generating relatively little light in the corals’ fluorescent emission spectra – such as green, yellow, orange or red wavelengths. In addition, there seems little doubt that ‘energetic’ wavelengths such as violet and blue can cause proteins to rearrange their molecular structures and thus shift from drab to colorful. But, as we shall see, there are other factors involved.)
Little distinction has been drawn by hobbyists (and many researchers for that matter) in the differences of required illumination (in captive conditions) for fluorescent and non-fluorescent proteins. Chromoproteins also seem to be expressed by the coral host in conditions of relatively high light energy skewed towards the higher kelvin wavelengths (violet, blue). Since chromoproteins are reflective by nature, the spectral characteristic of the lamp(s) illuminating the aquarium again takes on importance. As we have seen, most chromoproteins found within corals absorb light energy at ~570-580 nanometers (since they do not preferentially absorb blue and red wavelengths, they reflect it), often giving corals an apparent blue-purple coloration. High kelvin metal halide lamps are generally poor in producing a large amount of red light, hence corals containing non-fluorescent reflective proteins are best viewed using lamps with a spectral output balanced between blue and red wavelengths, such as 6,500 – 10,000K lamps.
In order to understand coral coloration, we must identify individual pigments. A fiber optic spectrometer can ‘see’ how light energy is absorbed, fluoresced or reflected. This article will examine reflected light and reflectance.
What is Reflectance?
Reflectance is the ratio of the total amount of radiation (such ultraviolet, visible and infrared wavelengths) reflected by a surface to the total amount of radiation incident to the surface. Reflectance is important when determining how an object absorbs and reflects light. In this manner, pigments can sometimes be identified.
Procedure
Use of a fiber optic spectrometer determines reflectance and fluorescence of different corals in a non-invasive manner. Two spectrometers were used to make these observations – a USB-2000 for reflectance and a USB-2000FL (especially configured for fluorescence measurements), both manufactured by Ocean Optics of Dunedin, Florida. A cosine-corrected collection lens and 600-micron fiber optic patch cord collected and delivered light to the spectrometer. Calculation of reflectance involves several steps: First, light is reflected from a calibrated standard (Spectralon, >99% diffuse reflection) using a standardized light source – in this case, a 100-watt clear glass tungsten filament incandescent lamp operating on a line voltage of 110v (under these conditions, this lamp is also known by the exotic name of ‘CIE Standard Illuminant A’, where the relative spectral power distribution is the approximately the same as a Planckian blackbody radiator at a temperature of about 2856 K – Hunt, 1998). Reflectance ratios were calculated by using Ocean Optic’s OOBase32 software. Figures 3, 4 and 5 illustrate how reflectance is determined.
Determination of Incident Light
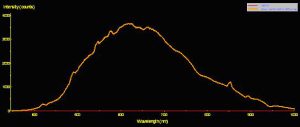
Figure 3. The spectral signature of a Standard Illuminant ‘A’ light source -a fancy name for a tungsten incandescent lamp.
Determination of Reflected Light
Reflectance
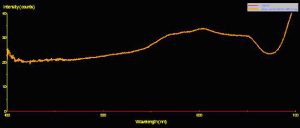
Figure 5. Reflectance is the ratio of reflected energy to incident energy it is an aid in understanding how corals/zooxanthellae utilize light energy. This is the reflectance of a brown Pocillopora meandrina. Note the spectral peaks at ~575nm, 600nm and 650nm (typical of many brown, green, yellow, orange or red corals).
What Reflectance Tells Us
In the grand scheme of things, reflectance is an important tool in coral reef remote monitoring via aircraft or satellite. In particular, seafloor reflectance allows large areas to be monitored with relative ease. Remote sensing is the subject of much research as it can identify coral bleaching and perhaps coral health, as well as discriminate between coral, algal and other benthic surfaces.

Figure 6. “Brown corals” possess a triple-peaked reflectance with peaks and shoulders at ~575, 600 and 650nm. This spectral signature is due to the presence of photosynthetic pigments within the symbiotic zooxanthellae (see Figure 7 below).
Some of this information is important to us as hobbyists. It should be noted that corals, as a group, reflect little light (as little as 0.5% at 400nm (violet), 2.5% at 400-500nm (blue through green), 8% between 550 and 650nm (green-red), and as much as ~100% at 700nm (red); Hochberg et al., 2004). However, these ratios are variable and seemingly small variations in the overall spectral signature of a coral can make a profound difference in how it appears to the human eye.
We should note that reflectance is not independent of fluorescence. Since fluorescent pigments often occur in corals, and will absorb light energy and emit it at less energetic wavelengths, their presence will impact the spectral reflectance. Careful examination of reflectance is required to distinguish between reflectance and fluorescence (something we’ll discuss in more detail later in this series).
Stony corals generally fall into two categories (as defined by Hochberg et al., 2000) – ‘brown’ corals and ‘blue’ corals. At face value, this seems odd – corals are known to exhibit a rainbow of colors. Let’s examine what these two terms mean.
‘Brown’ Corals
Generally, coral reflectance most often falls into this category. Reflectance of ‘brown’ corals generally exhibits a distinctive pattern, with peaks at 575, 600 and 600 nm (likely due to the wavelengths not absorbed -and hence reflected – by zooxanthellae). This ‘triple peak’ or ‘crown’ reflectance is also seen in corals exhibiting (in addition to brown) red, orange, yellow or green coloration (see Figures 6, 7 and 8).
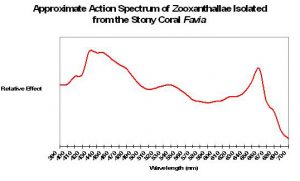
An ‘action spectrum’ shows the efficiency of light (by wavelength) in producing a photochemical reaction. If this image was inverted, it would resemble the reflectance shown in Figure 6 – light not absorbed is reflected! Absorption is due to the presence of photopigments including chlorophyll a, chlorophyll c2, peridinin and a few other minor pigments.
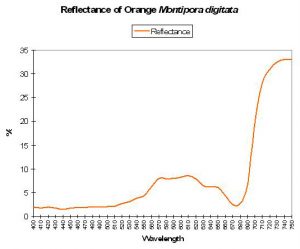
Figure 8. Reflectance of an orange coral – a variation of ‘brown coral’ reflectance – note the presence of the triple-peaked reflectance.
‘Blue’ Corals
‘Blue’ coral reflectance is the name given to a group of corals exhibiting purple, blue, pink or grey coloration, which is indicative of the possible presence of non-fluorescent proteins. ‘Blue’ corals lack the peak at ~575nm (as seen in ‘brown’ corals, and in close proximity to the maximum absorbance of many non-fluorescent proteins).
Transitional Coloration – Colors Are Not Always There
Although many researchers have categorized corals’ reflectance as either ‘brown’ or ‘blue’ there must surely be transitional states. Although much research needs to be done, we have some data showing the reflectance of genetically-identical coral fragments (See Figure 10). In this particular experiment, Acropora (austera? – commonly called the ‘Purple Monster’) fragments were placed in similar conditions with the exception of light intensity. The fragment in ‘low’ light (138 µmol·m²·sec) lost its mauve coloration over the period of a few weeks. The ‘high’ light fragment (maintained at 875µmol·m²·sec) retained its brilliant (but non-fluorescent) ‘purple’ coloration.

Figure 9. A spectral signature typical of ‘blue’ corals. This category includes purple, blue, pink or grey corals. Spectral reflectance from a ‘purple’ Cyphastrea specimen.
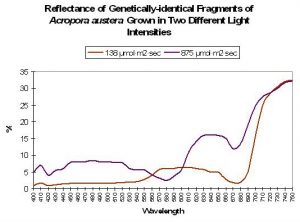
Figure 10. Colors are not ‘always there’ as demonstrated by the reflectance of genetically-identical coral fragments grown under ‘low’ and ‘high’ high intensities.
Pigment 580
At the time of this writing, Pigment 580 is the only chromoprotein I have been able to identify, and this was seen in an Acropora (austera? – see Figure 11).

Figure 11. The ‘Purple Monster’Acropora. It contains a non-fluorescent pigment with maximum absorption at 580nm; hence it preferentially reflects blue and red wavelengths thus making it appear ‘purple’.
Interestingly enough, Pigment 580 is likely a photo-activated pigment and has been described only in Acropora species, including A. aculeus and A. hyacinthus (Matz et al., 2005). However, note that a chromoprotein with maximum of 580nm has been described from Goniopora tenuidens (Martynov et al., 2003). This pigment can mature in darkness and its expression is probably not linked to any given light intensity. Although its color is similar to those pigments found in Acropora species, the Goniopora pigment is probably of an entirely different class of chromoproteins from those found in some Acropora specimens.
Where Are Corals Containing Chromoproteins Found in Nature?
Salih (2003) investigated how chromoproteins are distributed across depth on the Great Barrier Reef (see Figure 12). Interestingly, there appears to be a fairly strong correlation between shallow depth and chromoprotein abundance with corals containing these reflective pigments composing about 22% of all corals on the reef flat and forereef. (Also of interest is the fact that shallow tidepools did not contain many corals containing chromoproteins. Salih did not address this issue, but we can speculate that tidepool environments were not conducive to those coral species known to contain reflective pigments. These corals include Acropora, Pocillopora, Seriatopora, Montipora and a few other genera).
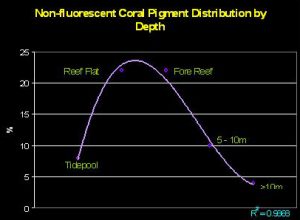
Figure 12. Water depth and its relationship to reflective coral pigments. The chart illustrates the percentage of corals containing non-fluorescent proteins across a transect line at various depths. After Salih, 2003.
The Apparent Effect of Light on Generating Coral Coloration
It seems apparent that light can be responsible for generating at least some colors within coral tissues. Riddle (2003) examined the apparent effects of spectral quality on coloration of the stony coral Pocillopora meandrina. Blue light (generated by monochromatic light-emitting diodes) apparently caused a color shift within the coral’s tissues from a brown color to non-fluorescent pink. We can speculate that the blue light induced expression of the pink pigment.
See http://www.advancedaquarist.com/issues/nov2003/feature.htm
‘Coloration Threshold’
The term ‘coloration threshold’ describes the point at which colorful coral pigments become apparent. Although many environmental factors must be proper (such as temperature, pH, etc.) in order for a color to ‘color up’, ‘coloration threshold’ will be used to describe the point at which light intensity is sufficient to induce expression of these pigments.
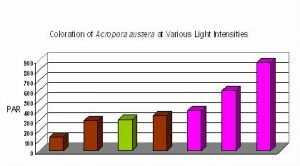
Figure 13. Coloration of Acropora austera specimens at different light intensities and spectra. The mauve coloration is not seen in this Acropora species until a light intensity of ~400 µmol·m²·sec is achieved. Interestingly, green coloration was noted at 310 µmol·m²·sec when the metal halide lamp was unshielded for ultraviolet radiation. Apparently, the strong UV encouraged the expression, or dominance, of the green fluorescent protein.
It seems certain that different light intensities are required for expression of various coral pigments. Some pigments, such as pink ‘pocilloporans’ are seen at relatively low light intensity. On the other hand, some pigments seem to require a great deal of light (at least in terms of aquaria illumination) for observation of colorful pigments, and such seems the case with the color of ‘Purple Monster’ Acropora specimens.
“Alkali Effect” on Chromoproteins
Battad et al., 2007 report that pH values above 9.0 can ‘kindle’ (that is, cause a chromoprotein to become a fluorescent protein) many coral pigments and cause dramatic (20-100 fold) increases in fluorescence efficiencies. Of course, we’re talking about corals’ tissue pH and not that of the ambient water.
Photosynthesis (such as that by corals’ symbiotic algae) is known to have an effect on pH. Removal of carbon dioxide (CO2) by algae will raise the pH during times of ‘sufficient’ illumination.
Kawaguti (1944) demonstrated that coral pigments changed colors in response to pH. This seminal researcher found Green Fluorescent Pigments (GFPs) reached a maximum intensity at pH 8 – 9. At higher and lower pH, the pigment was less intense but coloration changes were reversible when using certain chemicals (the same held true for pink and purple pigments, but not yellow. As a footnote, he found the GFP did not “bleach” when exposed to hydrogen peroxide – an oxygen radical believed by some today to destroy coral pigments). Gentien (1981) reported disappearance of blue fluorescence at pH 2 and, at pH 10, a shift in maximum fluorescence from 432 nm to 450. Interestingly, the maximum absorption by photoprotective pigments found in zooxanthellae (diadinoxanthin, diatoxanthin and beta-carotene) all have a maximum absorption in 445-451nm range. Are pH-induced shifts in coloration a small part of the arsenal against photo-damage and photo-destruction of zooxanthellae and coral animals?
This could very well be the case since the pH of photosynthetic invertebrates’ tissues and fluids are altered by rates of photosynthesis (that is, the removal or addition of carbon dioxide). Fitt et al. (1995) found diurnal pH values of 7.2 – 8.1 in Tridacna derasa and T. gigas tissues. Coral tissue pH demonstrate diurnal swings as well; Acropora and Favia tissue pH may be as high as 8.5 in light and fall rapidly to as low as 7.3 in darkness. Natural pH swings likely help regulate (or alter) the expression of coloration within coral tissues. In experiments with water-soluble and variously colored pigments extracted from corals, pH was lowered in the samples with CO² instead of caustic or acidic reagents used by Kawaguti (1944). Transmittance (as measured with a spectrophotometer) rose as the pH fell (personal observations). In other words, it seems that pH-modulated light transmission (along with shifts in fluorescence) could possibly help prevent photo-damage to zooxanthellae and hence the coral animal.
Temperature
Kawaguti (1944) also reported heat (boiling) could destroy some pigments, while other pigments (such as pocilloporins) may be stable at relatively high temperatures (Dove et al., 2001). The potential for loss of ‘photo-protective’ coloration due to high temperatures could have devastating effects on coral colonies and zooxanthellae.
That high temperature could denature (destroy), or at least alter the absorbance capacity of, pigment proteins with possible photoprotective abilities is a quite interesting thought, especially when considering temperatures apparent role in coral “bleaching.” Denaturing of proteins is often an irreversible process, such as the changes seen in an egg yolk when its proteins are heated and denatured. Colorful corals collected and frozen for later lab work do not lose their coloration (personal observation).
In general, reef aquarists are advised to keep water temperatures in the mid-to-high 70ºF range. Under certain conditions, it is possible for coral skeletons to act as heat-sinks and actually become slightly warmer than the ambient water temperature. This rather disturbing news (especially for those corals maintained in aquaria teetering on the edge of acceptable upper-scale temperatures) is discussed by Riddle, 2006 (see www.advanceaquarist.com/2006/2/aafeature2 ).
In Closing
Spectrometry and determination of reflectance allows us to investigate coral coloration at a level previously unknown within the hobby. Once we have identified the pigment(s) within a particular coral, we can then begin to understand the environmental conditions that are favorable to the expression of the pigment(s).
We must be careful to avoid the pitfall of thinking a single environmental parameter alone is responsible for coloration – many factors are synergistically linked. Although light certainly seems important, photosynthesis depends upon a source of inorganic carbon (such as bicarbonates – ‘alkalinity’ – or CO2) which in turn could be potentially limited by insufficient water flow. By the same token, CO2, due to coral and zooxanthellae respiration, could possibly accumulate within the stagnant boundary layer surrounding the coral and thus drive pH downwards. Temperature is probably less important to the coral animal than its resident zooxanthellae. However, loss of zooxanthellae could be catastrophic to the coral so, naturally, avoid temperatures approaching 80ºF or so. Carefully monitor any corals directly under metal halide lamps for loss of pigmentation due to ‘spot warming’ resulting in bleaching.
The only chromoprotein identified in an aquarium coral, so far, is Pigment 580. Currently, it is known to occur in only Acropora specimens. This chromoprotein seems to be expressed at relatively high light levels (~400 µmol·m²·sec) when other factors (such as those mentioned above) are correct.
Though only briefly discussed, hobbyists should abandon the thought that ultraviolet radiation is responsible and therefore necessary for inducing the expression of chromoproteins. It is apparent that violet and blue wavelengths can also cause coloration shifts. Excessive ultraviolet radiation usually isn’t a problem with fluorescent lamps and it is definitely not a problem with the newer LED luminaires. However, hobbyists should shield their metal halide lamps for UV radiation by using acrylic panes. For those doubters, I’ll present some information on the effects of UV on some fluorescent proteins in the next installment of this series.
As mentioned, some of this information was presented at MACNA XIX in Pittsburgh. For those who have never attended a national conference such as MACNA, there are many reasons you should do so. You’ll benefit from the presentations and be one of the first to see new products aimed at the reef aquaria market, but the real treat is the fellowship with 1,000 other hobbyists. Atlanta is hosting MACNA XX in September 2008. I hope to see you there! See www.masna.org for conference details and registration.
I wish to thank Steve Ruddy and Coral Reef Ecosystems (www.coralreefecosystems.com) for his help during the preparation of this and future articles. I simply could not have done it without his help.
Updated References and Suggested Reading
- Ai, H-w., J. Henderson, S. Remington, and R. Campbell, 2006. Directed evolution of monomeric, bright and photostable version of Clavularia cyan fluorescent protein: structural characterization and applications in fluorescent imaging. Biochem. J., 15, 400(Part 3): 531-540.
- American Public Health Association (APHA), 1998. Standard Methods for the Examination of Water and Wastewater. Edited by L. Cleceri, A. Greenberg and A. Eaton. Washington, DC.
- Anonymous. Advanced collection of fluorescent proteins. Evrogen Joint Stock Company, Moscow. www.evrogen.com
- Ando, R., H. Hama, M. Yamamoto-Hino, H. Mizuno, and A. Miyawaki, 2002. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. USA, 99(20):12651-12656.
- Ando, R., H. Mizuno and A. Miyawaki, 2004. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science, 306:1370-1373.
- Andréfouët, A., C. Payri, E. Hochberg, L. Che and M. Atkinson, 2003. Airborne hyperspectral detection of microbial mat pigmentation in Rangiroa atoll (French Polynesia). Limnol. Oceanogr., 48(1, Part 2):426-430.
- Andresen, M., M. Wahl, A. Stiel, F. Gräter, L. Schäfer, S. Trowitzsch, G. Weber, C. Eggeling, H. Grubmüller, S. Hell and S. Jakobs, 2005. Structure and mechanism of the reversible photoswitch of a fluorescent protein. Proc. Natl. Acad. Sci. USA, 102, 37:13070-13074.
- Apprill, A., 2003. Spectral characteristics and genetic expression of green fluorescent proteins in Hawaiian corals. In: Molecular Biology of Corals: Results of 2002 Edwin W. Pauley Summer Program in Marine Biology, E. Cox and T. Lewis, eds. University of Hawaii HIMB Technical Report No. 43:6-13.
- Apprill, A., R. Bidigare and R. Gates, 2007. Visibly healthy corals exhibit variable pigment concentrations and symbiont phenotypes. Coral Reefs,.
- Baird, G., D. Zacharias and R. Tsien, 2000. Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proc. Natl. Acad. Sci. USA, 97(22):11984-11989.
- Banaszak, A., T. LaJeunesse and R. Trench, 2000. The synthesis of mycosporine-like amino acids (MAAs) by cultured, symbiotic dinoflagellates. J. Exp. Mar. Biol. Ecol., 249: 219-233.
- Bandaranayake, W., 1998. Mycosporines: Are they nature’s sunscreens? National Product Review, 1998. 159-172.
- Battad, J., P. Wilmann, S. Olsen, E. Byres, S. Smith, S. Dove, K. Turcic, R. Devenish, J. Rossjohn and M. Prescott, 2007. A structural basis for the pH-dependent increase in fluorescence efficiency of chromoproteins. J. Mol. Biol.
- Beddoe, T., M. Ling, S. Dove, O. Hoegh-Guldberg, R. Devenish, M. Prescott and J. Rossjohn, 2003. The production, purification and crystallization of a pocilloporan pigment from a reef-forming coral. Acta Crystallographica, 59(3):597.
- Bingman, C., 1995. The effect of activated carbon treatment on the transmission of visible and UV light through aquarium water. I: Time-course of activated carbon treatment and biological effects. Aquarium Frontiers 2(3): 4,5,16-19.
- Bingman, C., 1995. Green-fluorescent protein: a model for coral host fluorescent proteins? Aquarium Frontiers, 2(3): 6-9.
- Bingman, C., 1999. Biochemistry of Aquaria: Coral Fluorescence – An Update. http://www.reefs.org/library/aquarium_frontiers/index.html
- Blundell, A., 2005. Lateral Lines: The Seen and Unseen World of Coral Fluorescence. http://www.advancedaquarist.com/2005/2/lines/
- Bonsma, S., R. Purchase, S. Jezowski, J. Gallus, F. Konz and S. Volker, 2005. Green and red fluorescent proteins: photo- and thermally induced dynamics probed by site-selective spectroscopy and hole burning. Chemphyschem, 6(5):838-849.
- Bulina, M., D. Chudakov, N. Mudrik, and K. Lukyanov, 2002. Interconversion of Anthozoa GFP-like fluorescent and non-fluorescent proteins by mutagenesis. BMC Biochem., 24; 3(1):7.
- Bulina, M., K. Lukyanov, I. Yampolsky, D. Chudakov, D. Staroverov, A. Shcheglov, N. Gurskaya and S. Lukyanov, 2004. New class of blue animal pigments based on frizzled and kringle protein domains. J. Biol. Chem., 279(42):43367-43370.
- Burr, A., P. Hunt, D. Wagar, S. Dewilde, M. Blaxter, J. Vanfleteren and L. Moens, 2000. A hemoglobin with an optical function. J. Biol. Chem., 275(7): 4810-4815.
- Calfo, A., 2005. Magnificent fluorescence! Aquaristic perspectives. http://reefkeeping.com/issues/2005-11/ac/index.php
- Carter, R., M. Schmale and P. Gibbs, 2004. Cloning of anthozoan fluorescent protein genes. Comp. Biochem. Physiol. C. Toxicol. Pharmacol., 138(3): 259-270.
- Chalfie, M. and S. Kain (eds.), 2006. Green Fluorescent Protein: Properties, Applications and Protocols, 2nd Edition. Methods of Biochemical Analysis, Vol. 47. John Wiley and Sons, Hoboken.
- Chan, M., S. Karasawa, H. Mizuno, I. Bosanac, D. Ho, G. Privé, A. Miyawaki and A. Ikura, 2006. Structural characterization of a blue chromoprotein and its yellow mutant from the sea anemone Cnidopus japonicus. J. Biol. Chem., 281(49): 37813-37819.
- Chattoraj, M., B. King, G. Bublitz and S. Boxer, 1996. Ultra-fast excited state dynamics in green fluorescent protein: Multiple states and proton transfer. Proc. Natl. Acad. Sci. USA, 93: 8362-8367.
- Chudakov, D., A. Feofanov, N. Mudrik, S. Lukyanov and K. Lukyanov, 2003. Chromophore environment provides clue to “kindling fluorescent protein” riddle. J. Biol. Chem., 278(9):7215-7219.
- Chudakov, D., V. Belousov, A. Zararisky, V. Novoselov, D. Staroverov, D. Zorov, S. Lukyanov and K. Lukyanov, 2003. Kindling fluorescent proteins for precise in vivo photolabeling. Nat. Biotechnol. 21(2): 191-194.
- Chudakov, D., V. Verkhusha, D. Staroverov, E. Souslova, S. Lukyanov, and K. Lukyanov, 2004. Photoswitchable cyan fluorescent protein for protein tracking. Nat. Biotechnol., 22(11):1435-1439.
- Cotlet, M., J. Hofkens, S. Habuchi, G. Dirix, M. Van Guyse, J. Michiels, J. Vanderleyden and F. De Schryver, 2001. Identification of different emitting species in the red fluorescent protein DsRed by means of ensemble and single-molecule spectroscopy. Proc. Natl. Acad. Sci. USA, 98(25):14398-14403.
- Cox, G. and A. Salih, 2005. Fluorescent lifetime imaging of symbionts and fluorescent proteins in reef corals. In: Multiphoton Microscopy in the Biomedical Sciences V, edited by A. Periasami and Peter So. Proc. SPIE, 5700:162-170.
- Credabel, J., 2006. Notes from the trenches: Coral fusion and grafting. http://reefkeeping.com/issues/2006-02/nftt/index.php
- D’Elia, C., S. Domotor and K. Webb, 1983. Nutrient uptake kinetics of freshly isolated zooxanthellae. Mar. Biol., 75:157-167.
- Dai, M., H. Fisher, J. Temirov, C. Kiss, M. Phipps, P. Pavlik, J. Werner and A. Bradbury, 2007. The creation of a novel fluorescent protein by guided consensus engineering. PEDS.
- Delbeek, J. and J. Sprung, 1994. The Reef Aquarium: A Comprehensive Guide to the Identification and Care of Tropical Marine Invertebrates. Vol. 1. Ricordea Publishing, Coconut Grove, Fl. 544 pp.
- Delbeek, J. and J. Sprung, 2005. The Reef Aquarium: Science, Art and Technology. Vol. 3. Ricordea Publishing, Coconut Grove, Fl. 679 pp.
- Delbeek, J.C., 2003. Media Review, Advanced Aquarist Online Magazine, June. www.advancedaquarist.com/issues/june2003/media.htm
- Diffley, A., Green fluorescent proteins in corals. Unpublished.
- Dittrich, P., S. Schäfer and P. Schwille, 2005. Characterization of the photoconversion on reaction of the fluorescent protein Kaede on the single-molecule level. Biophys. J., 89:3446-3455.
- Dove, S., M. Takabayashi and O. Hoegh-Guldberg, 1995. Isolation and partial characterization of the pink and blue pigments of Pocilloporid and Acroporid corals. Biol. Bull., 189:288-297.
- Dove, S., O. Hoegh-Guldberg and S. Ranganathan, 2001. Major color patterns of reef-building corals are due to a family of GFP-like proteins. Coral Reefs 19: 197-204.
- Dove, S., 2004. Scleractinian corals with photoprotective host pigments are hypersensitive to thermal bleaching. Mar. Ecol. Prog. Ser., 272: 99-116.
- Dove, S., J. Oritz, S. Enriquez, M. Fine, P. Fisher, R. Iglesias-Prieto, D. Thornhill and O. Hoegh-Guldberg, 2006. Response of holosymbiont pigments from the scleractinian coral Montipora monasteriata to short-term heat stress. Limnol. Oceanogr., 51(2): 1149-1158.
- Dunlap, W.C. and B.E. Chalker, 1986. Identification and quantification of near-UV absorbing compounds (S-320) in a hermatypic scleractinian. Coral Reefs, 5:155-159.
- Elowitz, M., M. Surette, P. Wolf, J. Stock and S. Leibler, 1997. Photoactivation turns green fluorescent protein red. Curr. Biol., 7(10):809-812.
- Evrogen Fluorescent Proteins, Advanced collection of fluorescent proteins.
- www.evrogen.com
- Fabricius, F., 2006. Effects of irradiance, flow and colony pigmentation on the temperature microenvironment around corals: Implications for coral bleaching. Limnol. Oceanogr., 51(1): 30-37.
- Field, S., M. Bulina, I. Kelmanson, J. Bielawski and M. Matz, 2006. Adaptive evolution of multicolored fluorescent proteins in reef-building corals. J. Mol. Evol., 62(6):332-339.
- Finet, B. and F. Lesage, 2005. Colors by the thousands – Light, Colors and Corals, Part 1.Advanced Aquarist Online, December 2005. http://www.advancedaquarist.com/2005/12/aafeature2
- Fischer, A., W. Coleman, M. Yang and J. Lagarias, 2004. Engineering phytochromes: biliproteins that switch and glow. Genetically Engineered and Optical Probes for Biomedical Applications, II: A. Savitsky, L. Brovko, D. Bornhop, R. Raghavachari, S. Achilefu, Editors. Proc. SPIE, Vol. 5329: 33-43.
- Fitt, W.K., T.A.V. Rees and D. Yellowlees, 1995. Relationship between pH and the availability of dissolved inorganic nitrogen in the zooxanthella-giant clam symbiosis. Limnol. Oceanogr., 40(5): 976-982.
- Fluorescence Applications in Biotechnology and Life Sciences. www.physics.mq.edu.au/research/fluoronet/
- Fox, D.L. and D.W. Wilkie, 1970. Somatic and skeletally fixed carotenoids of the purple hydrocoral, Allopora californica. Comp. Biochem. Physiol., 36:49-60.
- Fox, D.L., 1972. Pigmented calcareous skeletons of some corals. Comp. Biochem. Physiol., 43B:919-927.
- Fux, E. and C. Mazel, Unpublished. An experimental method to separate the fluorescence and reflectance components of the spectral signatures of corals.
- Fux, E. and C. Mazel, 1999. Unmixing coral fluorescence emission spectra and predicting new spectra under different excitation conditions. Applied Optics. 38, 3: 486-494.
- Garcia-Parajo, M., M. Koopman, E. van Dijk, V. Subramaniam, and N.F. van Hulst, 2001. The nature of fluorescence emission in the red fluorescent protein DsRed, revealed by single-molecule detection. Proc. Natl. Acad. Sci. USA, 98(25):14392-14397.
- Gentien, P., 1981. Fluorescent metabolites in coral reefs off Townsville, Queensland. Aust. J. Mar. Freshwater Res., 32: 975-980.
- Gilmore, A., A. Larkum, A. Salih, S. Itoh, Y. Shibata, C. Bena, H. Yamasaki, M. Papina and R. van Woesik, 2003. Simultaneous time resolution of the emission spectra of fluorescent proteins and zooxanthellar chlorophyll in reef-building corals. Photochem. Photobiol., 77(5): 515-523.
- Gorbunov, M. and P. Falkowski, 2002. Photoreceptors in the cnidarian hosts allow symbiotic corals to sense blue moonlight. Limnol. Oceanogr., 47(1): 309-315.
- Gross, L., G. Baird, R. Hoffman, K. Baldridge and R. Tsien, 2000. The structure of the chromophore with DsRed, a red fluorescent protein from coral. Proc. Natl. Acad. Sci. USA, 97(22): 11990-11995.
- Gulko, D., M. Lesser and M. Ondrusek, 1995. Introduction of materials and methods commonly used by participants in the 1994 HIMB summer program on UV radiation and coral reefs. . In: Ultraviolet Radiation and Coral Reefs. D. Gulko and P.L. Jokiel (eds.), HIMB Technical Report #41, 19-23.
- Gurskaya, N., A. Fradkov, A. Terskikh, M. Matz, Y. Labas, V. Martynov, Y. Yanushevich, K. Lukyanov and S. Lukyanov, 2001. GFP-like chromoproteins as a source of far-red fluorescent proteins. FEBS Letters, 507(1):16-20.
- Gurskaya, N., V. Verkhusha, A. Shcheglov, D. Staroverov, T. Chepurnykh, A. Fradkov, S. Lukyanov and K. Lukyanov, 2006. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nature Biotechnology, 24: 461-465.
- Hanson, G. T. McAnaney, E. Park, M. Rendell, D. Yarbrough, S. Chu, L. Xi, S. Boxer, M. Montrose and S. Remington, 2002. Green fluorescent protein variants as ratiometric dual emission pH sensors. 1. Structural characterization and preliminary application. Biochemistry, 41(52):15477-88.
- Hedley, J. and P. Mumby, 2002. Biological and remote sensing perspectives of pigmentation in coral reef organisms. Adv. Mar. Biol., 43:277-317.
- Heim, R., D. Prashner and R. Tsien, 1994. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc. Natl. Acad. Sci. USA, 91: 12501-12504.
- Henderson, J. and S. Remington, 2005. Crystal structures and mutational analysis of amFP486, a cyan fluorescent protein from Anemonia majano. Proc. Natl. Acad. Sci. USA, 102, 36: 12712-12717.
- Hochberg, E. and M. Atkinson, 2000. Spectral discrimination of coral reef benthic communities. Coral Reefs, 19:164-171.
- Hochberg, E., M. Atkinson, A. Apprill and S. Andrèfouët, 2004. Spectral reflectance of coral. Coral Reefs, 23: 84-95.
- Hochberg, E., A. Apprill, M. Atkinson and R. Bidigare, 2006. Bio-optical modeling of photosynthetic pigments in corals. Coral Reefs, 25: 99-109.
- Hollingsworth, L., R. Kinzie III, T. Lewis, D. Krupp and J-AC Leong, 2005. Photoaxis of motile zooxanthellae to green light may facilitate symbiont capture by coral larvae. Coral Reefs, 24: 523.
- Hunt, R., 1998. Measuring Colour. Fountain Press, Kingston-upon-Thames, England. 344 pp.
- Ianushevich, I., N. Gurskaia, D. Staroverov, S. Lukyanov and K. Lukyanov, 2003. A natural fluorescent protein that changes its fluorescence during maturation. Bioorg Khim., 29(4):356-360.
- Ip, D., S-H Chan, M. Allen, M. Bycroft, D. Wan and K-B Wong, 2004. Crystallization and preliminary crystallographic analysis of a novel orange fluorescent protein from the cnidaria tube anemone Cerianthus. Acta Crystallogr., Section D, 60:340-341.
- Israel, A. and S. Beer, 1992. Photosynthetic carbon acquisition in the red alga Gracilaria conferta. 2. Rubisco carboxylase kinetics, carbonic anhydrase, and bicarbonate uptake. Mar. Biol., 112:697-700.
- Jeffrey, S., R. Mantoura and S. Wright, eds., 1997. Monographs on Oceanographic Methodology: Phytoplankton Pigments in Oceanography. UNESCO Publishing, France. 661 pp.
- Karan, M., B. Filippa, J. Mason, S. Dove, O. Houegh-Guldberg and M. Prescott, 2006. Cell visual characterisitic-modifying sequences. US Patent US2006/0107351 A1.
- Karasawa, S., T. Araki, M. Yamamoto-Hino, and A. Miyawaki, 2003. A green-emitting fluorescent protein from Galaxeidae coral and its monomeric use in fluorescent labeling. J. Biol. Chem., 278:34167-34171.
- Karasawa, S., T. Araki, T. Nagai, H. Mizuno and A. Miyawaki, 2004. Cyan-emitting and orange-emitting fluorescent proteins as a donor/acceptor pair for fluorescent resonance energy transfer. Biochem. J., 381(Part 1):307-312.
- Khang, S. and A. Salih, 2005. Localization of fluorescent pigments in a nonbioluminescent, azooxanthellate octocorals suggests a photoprotective function. Coral Reefs, 24, 3: 435.
- Kawaguti, S., 1937. On the physiology of reef corals II. The effect of light on colour and form of reef corals. Palao Trop. Biol. Sta. Taihoku Imperial University, Taihoku. 2.
- Kawaguti, S., 1944. On the physiology of reef corals VI. Study on the pigments. Palao Trop. Biol. Sta. Study, 2:617-674.
- Kawaguti, S., 1966. Electron microscopy on the fluorescent green of reef corals with a note on the mucous cells. Biol. J. Okayama Univ., 12:11-21.
- Kelmanson, I. and M. Matz, 2003. Molecular basis and evolutionary origins of color diversity in Great Star coral Montastraea cavernosa (Scleractinia:Faviida). Mol. Biol. Bull., 20, 7: 1125-1133.
- Kennedy, G.Y., 1979. Pigments of marine invertebrates. Adv. Mar. Biol., 16:309-381.
- Kinzie, R.A. and T. Hunter, 1987. Effect of light quality on photosynthesis of the reef coral Montipora verrucosa. Mar. Biol., 94:95-109.
- Kinzie, R.A., P.L. Jokiel and R. York, 1984. Effects of light of altered spectral composition on coral zooxanthellae associations and on zooxanthellae in vitro. Mar. Biol., 78:239-248.
- Kirk, J.T.O., 1983. Light and Photosynthesis in Aquatic Ecosystems. Cambridge University Press, Cambridge. 401 pp.
- Koh, E., 1997. Secretion of bioactive compounds by a scleractinian coral. Proc. 8th Int. Coral Reef Symp., 2:1263-1266.
- Kuffner, I.B., M.E. Ondrusek and M.P. Lesser, 1995. Distribution of mycosporine-like amino acids in the tissues of Hawaiian scleractinia: a depth profile. In: Ultraviolet Radiation and Coral Reefs. D. Gulko and P.L. Jokiel, Eds. HIMB Tech. Report #41.
- Kutser, T., A. Dekker and W. Skirving, 2003. Modeling spectral discrimination of Great Barrier Reef benthic communities by remote sensing instruments. Limnol. Oceanogr., 48(1, Part 2): 497-510.
- Labas, Y., N. Gurskaya, Y. Yanushevich, A. Fradkov, K. Lukyanov, S. Lukyanov, and M. Matz, 2002. Diversity and evolution of the green fluorescent protein family. Proc. Natl. Acad. Sci. USA. 99 (7): 4256-4261.
- Larkum, A., G. Cox, R. Hiller, D. Parry and T. Dibbayawan, 1987. Filamentous cyanophytes containing phycocourobilin and in symbiosis with sponges and an ascidian of coral reefs. Mar. Biol., 95(1):1-13.
- Lesser, M., C. Mazel, D. Phinney and C. Yentsch, 2000. Light absorption and utilization by colonies of the congeneric hermatypic corals Montastraea faveolata and Montastraea cavernosa. Limnol. Oceanogr., 45(1): 76-86.
- Leutenegger, A., C. D’Angelo, M. Matz, A. Denzel, F. Oswald, A. Salih, G. Nienhaus and J. Wiedenmann, 2007. It’s cheap to be colorful: Anthozoans show a slow turnover of GFP-like proteins. FEBS Journ., 274(10):2496-2505.
- Living Colors User Manual, Volume 2. Reef Coral Fluorescent Proteins. www.clonetech.com
- Logan, A., K. Halcrow and T. Tomascik, 1990. UV excitation-fluorescence in polyp tissue of certain scleractinian corals from Barbados and Bermuda. Bull. Mar. Sci., 46(3):807-813.
- Louchard, E., R. Reid, C. Stephens, C. Davis, R. Leathers and T. Downes, 2003. Optical remote sensing of benthic habitats and bathymetry in coastal environments at Lee Stocking Island, Bahamas: A comparative spectral classification approach. Limnol. Oceanogr., 48(1):511-521.
- Lukyanov, K., A. Fradkov, N. Gurskaya, M. Matz, Y. Labas, A. Savitsky, M. Markelov, A. Zaraisky, X. Zhao, Y. Fang, W. Tan and S. Lukyanov, 2000. Natural animal coloration can be determined by a non-fluorescent green fluorescent protein homolog. J. Biol. Chem., 275(34):25879-25882.
- Lukyanov, K., D. Chudakov, S. Lukyanov and V. Verkhusha, 2005. Photoactivatable fluorescent proteins. Nature Rev. Mol. Cell Biol., 6:885-891.
- Manica, A. and R. Carter, 2000. Morphological and fluorescent analysis of the Montastraea annularis species complex in Florida. Mar. Biol., 137(5-6):899-906.
- Martynov, V., B. Maksimov, N. Martynova, A. Pakhomov, N. Gurskaya and S. Lukyanov, 2003. A purple-blue chromoprotein from Goniopora tenuidens belongs to the DsRed subfamily of GFP-like proteins. J. Biol. Chem., 278(47):46288-46292.
- Martynov, V., A. Savitsky, N. Martynov, P. Savitsky, K. Lukyanov and S. Lukyanov, 2001. Alternative cyclization in GFP-like proteins family. The formation and structure of the chromophore of a purple chromoprotein from Anemonia sculata. J. Biol. Chem., 276(24):21012-21016.
- Matz, M., A. Fradkov, Y. Labas, A. Savitsky, A. Zaraisky, M. Markelov and S. Lukyanov, 1999. Fluorescent proteins from nonbioluminescent Anthozoa species. Nature Biotechnology, 17:969-973.
- Matz, M., 2002. Diversity and evolution of the green fluorescent protein family. Proc. Natl. Acad. Sci. USA. 99 (7): 4256-4261.
- Matz, M., K. Lukyanov and S. Lukyanov, 2002. Family of the green fluorescent protein: Journey to the end of the rainbow. Bioessays 24(10):953-959.
- Matz, M., N. Marshall and M. Vorobyev, 2006. Are corals colorful? J. Am. Soc. Photochem. Photobiol., 82(2): 345-350.
- Matz, M., I. Kelmanson, E. Meleshkevitch and A. Salih, 2005. Novel fluorescent and colored proteins, and polynucleotides that encode these proteins. US Patent US 2005/0048609 A1.
- Mazel, C.H., 1995. Spectral measurements of fluorescence emission in Caribbean cnidarians. Mar. Ecol. Prog. Ser., 120:185-191.
- Mazel, C.H., 1997. Coral fluorescence characteristics: excitation – emission spectra, fluorescence efficiencies, and contribution to apparent reflectance. Ocean Optics XIII. 240-245.
- Mazel, C., M. Lesser, M. Gorbunov, T. Barry, J. Farrell, K. Wyman and P. Falkowski, 2003. Green fluorescent proteins in Caribbean corals. Limnol. Oceanogr., 48(1, part 2), 402-411.
- Mazel, C., M. Lesser, M. Gorbunov and P. Falkowski, 2004. Discovery of nitrogen-fixing cyanobacteria in corals. Science, 305: 997-1000.
- Mazel, C. H., and E. Fuchs, 2003. Contribution of fluorescence to the spectral signature and perceived color of corals. Limnol. Oceanogr. 48:390-401.
- Mazel, C.H., 2005. Underwater fluorescence photography in the presence of ambient light. Limnol. Oceanogr.: Methods 3:499-510.
- Mazel, C. Coastal Benthic Optical Properties (CoBOP): Optical properties of benthic marine organisms and substrates. www.psicorp.com/cobop
- Minghelli-Roman, A., J. Chisolm, M. Marchioretti, and J. Jaubert, 2002. Discrimination of coral reflectance spectra in the Red Sea. Coral Reefs, 21(3):307-314.
- Miyawaki, A., 2002. Green fluorescent protein-like proteins in reef Anthozoa animals. Cell Struct. Func., 27:343-347.
- Miyawaki, A., A. Sawano and T. Kogure, 2003. Lighting up cells: labeling proteins with fluorophores. Nature Reviews,Imaging in Cell Biology. S1-S7.
- Moore, L. and S. Chisolm, 1999. Photophysiology of the marine cyanobacterium Prochlorococcus: Ecotypic differences among cultured isolates. Limnol. Oceanogr., 44(3):628-638.
- Moseley, H., 1877. On the colouring matter of various animals and especially of deep-sea forms dredged by the H.M.S. Challenger. Quarterly Journal of the Microscopical Society, 17: 1-23.
- Mouget, J.-L., G. Tremblin, A. Morant-Manceau, M. Morançais and J.-M. Robert, 1999. Long-term photoacclimation of Haslea ostrearia (Bacillariophyta): effect of irradiance on growth rates, pigment content and photosynthesis. Eur. J. Phycol., 34(2):109-115.
- Myers, M., J. Hardy, C. Mazel, and P. Dustan, 1999. Optical spectra and pigmentation of Caribbean reef corals and macroalgae. Coral Reefs 18: 2, 179-186.
- Nienhaus, K., B. Vallone, F. Renzi, J. Wiedenmann and G. Nienhaus, 2003. Crystallization and preliminary X-ray diffraction analysis of the red fluorescent protein eqFP611. Acta Cryst., D59:1253-1255.
- Nienhaus, K., F. Renzi, B. Vallone, J. Wiedenmann and G. Nienhaus, 2006. Chromophore-protein interactions in the anthozoan green fluorescent protein asFP499. Biophys. J., 91:4210-4220.
- Nienhaus, K., G. Nienhaus, J. Wiedenmann and H. Nar, 2005. Structural basis for photo-induced protein cleavage and green-to-red conversion of fluorescent protein EosFP. Proc. Natl. Acad. Sci., USA, 102(26):9156-9159.
- Neveux, J., M. Tenório, C. Dupouy and T. Villareal, 2006. Spectral diversity of phycoerythrins and diazotroph abundance in tropical waters. Limnol. Oceanogr., 51(4):1689-1698.
- Ostergaard, H., A. Henriksen, F. Hansen, and J. Winther, 2001. Shedding light on disulfide bond formation: a redox switch in green fluorescent protein. EMBO J., 20(21):5853-5862.
- Oswald, F., F. Schmitt, A. Leutenegger, S. Ivanchenko, C. D’Angelo, A. Salih, S. Maslakova, M. Bulina, R. Schirmbeck, G. Nienhaus, M. Matz and J. Wiedenmann, 2007. Contributions of host and symbiont pigments to the coloration of reef corals. FEBS J., 274: 1102-1122.
- Pakahomov, A., N. Martynova, N. Gurskaya, T. Balashova and V. Martynov, 2004. Photoconversion of the chromophore of a fluorescent protein from Dendronephthya sp. Biochem. (Mosc.), 69(8):901-908.
- Papina, M., Y. Sakihama, C. Bena, R. van Woesik and H. Yamasaki, 2002. Separation of highly fluorescent proteins by SDS-PAGE in Acroporidae corals. In press, Comp. Biochem. Physiol.
- Paringit, E. and K. Nadaoka, 2001. Development of a canopy reflectance model for coral reef areas: Inferences from field spectral measurements and modeling efforts. Proc. 22nd Asian Conference on Remote Sensing.
- Pasternak, Z., B. Blasius, A. Abelson and Y. Achituv, 2006. Host-finding behavior and navigation capabilities of symbiotic zooxanthellae. Coral Reefs, 25(2):201-207.
- Patterson, G., R. Day and D. Piston, 2001. Fluorescent protein spectr. J. Cell Sci., 114:837-838.
- Pederson, H., 1968. Fluorescent flowers of the sea. Sea Frontiers, 14(4): 194-199.
- Peelle, B., T. Gururaja, D. Payan and D. Anderson, 2001. Characterization and use of green fluorescent proteins from Renilla mulleri and Ptilosarcus guernyi for the human cell display of functional peptides. J. Protein Chem., 20(6):507-19.
- Pieribone, V. and D. Gruber, 2005. Aglow in the Dark: The Revolutionary Science of Biofluorescence. The Belknap Press of Harvard University Press, Cambridge Massachusetts, and London, England. 263 pp.
- Piniak, G., N. Fogarty, C. Addison and W. Kenworthy, 2005. Fluorescence census techniques for coral recruits. Coral Reefs, 24: 496-500.
- Pouvreau, J.-B., M. Morançais, F. Fleury, P. Rosa, L. Thion, B. Cahingt, F. Zal, J. Fleurence and P. Pondaven, 2006. Preliminary characterization of the blue-green pigment “marennine” from the marine tychopelagic diatom Haslea ostrearia (Gaillon/Bory) Simonsen. J. Appl. Phycol., 18:757-767.
- Prashner, D., V. Enckenrode, W. Ward, F. Pentegast and M. Cormier, 1992. Primary structure of the Aequorea victoria green-fluorescent protein. Gene, 111: 229-233.
- Prescott, M., 2007. Internet PowerPoint presentation.
- Rahimi, Y., S. Shrestha and S. Deo, 2007. Metal affinity-based purification of a red fluorescent protein. Chromotographia, 65(7-8):429-433.
- Riddle, D. and A. Amussen, 1998. Coral pigments. Online presentation. http://www.reefs.org/library/talklog/d_riddle_042698.html
- Riddle, D., and A. Amussen, 1999. Coral Coloration, Part 1. Freshwater and Marine Aquarium, 22(12):34-40.
- Riddle, D., and A. Amussen, 2000. Coral Coloration, Part 2. Freshwater and Marine Aquarium, 23(1):80-82.
- Riddle, D., 2001. Farbpigmentation von steinkorallen. Koralle, 7:34-38.
- Riddle, D., 2003. Effects of narrow bandwidth light sources on coral host and zooxanthellae pigments. Advanced Aquarist Online, November 2003. http://www.advancedaquarist.com/issues/nov2003/feature.htm
- Riddle, D., 2004. Playing with poison – Ultraviolet radiation. http://advancedaquarist.com/issues/aug2004/feature.htm
- Riddle, D., 2006. Temperature and the reef aquarium. http://advancedaquarist.com/2006/2/aafeature2
- Riddle, D., 2006. Product Review: Ocean Optics spectrometers and software. Advanced Aquarist Online, June 2006. http://www.advancedaquarist.com/2006/6/review
- Riddle, D., 2006. Coral coloration, Fluorescence. Part 1. Advanced Aquarist Online, September 2006. http://www.advancedaquarist.com/2006/9/aafeature
- Riddle, D., 2006. Coral coloration, Fluorescence. Part 2. Pigments 510-565 and notes on GFPs. Advanced Aquarist Online, November 2006. http://www.advancedaquarist.com/2006/11/aafeature2
- Riddle, D., 2006. Coral coloration, Fluorescence. Part 3. Pigments responsible for yellow and orange coloration, with notes on photoconversion. Advanced Aquarist Online, December 2006. http://www.advancedaquarist.com/2006/12/aafeature2
- Riddle, D., 2007. Coral coloration, Fluorescence. Part 4. Red fluorescent pigments, with a preliminary report on effects of various environmental conditions and color mixing. Advanced Aquarist Online, February 2007. http://www.advancedaquarist.com/2007/2/aafeature
- Riddle, D., 2007. Coral coloration, Chromoproteins, Part 5. AdvancedAquarist Online. In press.
- Riddle, D., 2007. Coral coloration, Chromoproteins, Part 6. AdvancedAquarist Online. In press.
- Rinkevich, B. and Y. Loya, 1983. Intraspecific competition networks in the Red Sea coral Stylophora pistillata. Coral Reefs, 1:161-172.
- Robert, J.-M., M. Morançais, E. Pradier, J.-L. Mouget and G. Tremblin, 2002. Extraction and quantitative analysis of the blue-green pigment “marennine” synthesized by the diatom Haslea ostrearia. J. Appl. Phycol., 14: 299-305.
- Sacchetti, A., V. Cappetti, C. Crescenzi, N. Celli, D. Rotilio and S. Alberti, 2004. Curr. Biol., 9(11):R391-393.
- Schäfer, S., P. Dittrich, E. Petrov and P. Schwille, 2006. Single molecule fluorescence imaging of the photoinduced conversion and bleaching behavior of the fluorescent protein Kaede. Micro. Res. Tech., 69(3):210-219.
- Salih, A., G. Cox, R. Syymczak, S. Coles, A. Baird, A. Dunstan, G. Cocco, J. Mills and A. Larkum, 2006. The role of host-based color and fluorescent pigments in photoprotection and in reducing bleaching stress in corals. Proc. 10th Coral Reef Symp., Okinawa, Japan. www.coralcoe.org.au/research/publications/Salih_ICRS_2006.pdf
- Salih, A., 2003. An exploration of light regulating pigments of reef-building corals from macro- to micro- and nano-scales. In: From Zero to Infinity, J. Nicholls and B. Pailthorpe, eds. The Science Foundation for Physics, University of Sydney. Chapter 4:49-69.
- Salih, A., A. Larkum, T. Cronin, J. Wiedenmann, R. Szymczak and G. Cox, 2004. Biological properties of coral GFP-type proteins provide clues for engineering novel optical probes and biosensors. In: Genetically Engineered and Optical Probes for Biomedical Applications II, A. Savitsky et al., eds. Proc. of SPIE, 5329:61-72.
- Salih, A., O. Hoegh-Guldberg and G. Cox, 1998. Photoprotection of symbiotic dinoflagellates by fluorescent pigments in reef corals. Proc. Australian Coral Reef Society, 75th Anniversary Conference, Heron Island, Australia. 217-230.
- Salih, A., A. Larkum, G.Cox, M.Kuhl and O.Hoegh-Guldberg, 2000. Fluorescent pigments in corals are photoprotective. Nature, 408: 850-853.
- Salih A, Larkum AWD & G Cox, 2001. Photoprotection from photoinhibition of symbiotic algae in corals by fluorescent pigments. 12th International Congress on Photosynthesis, Brisbane, Australia.
- Salih, A., G. Cox and A. Larkum, 2003. Cellular organization and spectral diversity of GFP-like proteins in live coral cells studied by single and multiphoton imaging and microspectroscopy. In: Multiphoton Microscopy in the Biomedical Sciences III, A. Periasamy and P. So, Editors. Proc. SPIE, Vol. 4963: 194-200.
- Salih, A, Larkum AWD & G Cox, 2001. Corals use fluorescent pigments as natural sunscreens. Australian Coral Reef Society Conference, Magnetic Island, Australia.
- Salih, A, Cox G & AWD Larkum, 2000. The role of fluorescent pigments: evidence of enhanced resistance to bleaching in fluorescently pigmented corals. IX International Coral Reef Symposium, Bali, Indonesia.
- Salih, A., G. Cox & AWD Larkum, 2000. Energy dissipation by fluorescence coupling by coral fluorescent pigments. Optical Society Conference, Application of Optical Techniques in Biological Sciences.
- Salih, A., Hoegh-Guldberg O, Cox G & AWD Larkum, 1999. Protection against bleaching of corals by fluorescent pigments during the 1998 mass bleaching event. XIX Pacific Science Congress.
- Salih A., Hoegh-Guldberg, O. & Cox G., 1999. Are fluorescent colors in corals photoprotective? Australian Coral Reef Society Conference.
- Salih, A., Hoegh-Guldberg, O. & Cox G., 1998. Do fluorescent pigments in corals provide photoprotection to their algal endosymbionts and reduce the severity of bleaching? Australian Coral Reef Society Conference.
- Salih, A., Hoegh-Guldberg O & Cox G., 1997. Bleaching responses of corals: the effects of light and elevated temperature on their morphology and physiology. Australian Coral Reef Society Conference.
- Salih, A., Hoegh-Guldberg O & Cox G., 1997. Photoprotection of symbionts by fluorescent sunscreens in reef corals. Australian Coral Reef Society 75th Anniversary Conference.
- Salih, A. and M. Matz, 2006. Photoactivatible chromo/fluorescent GFP-like proteins and imaging applications. International Patent WO/2007/019382.
- Salih, A., 2005. Novel GFP-like proteins from reef corals with unique light-inducible properties highly suited for fluorescence imaging technologies. Micrscop. Micranal., 11(2).
- Schlichter, D., W. Weber and H.W. Fricke, 1985. A chromatophore system in the hermatypic, deep water coral Leptoseris fragilis (Anthozoa: Hexacorallia). Mar. Biol. 89:143-147.
- Schlichter, D., H.W. Fricke and W. Weber, 1986. Light harvesting by wavelength transformation in a symbiotic coral of the Red Sea twilight zone. Mar. Biol., 91:403-407.
- Schonwald, H., Y. Achituv, and Z. Dubinsky, 1987. Differences in the symbiotic interrelation in dark and light coloured colonies of the hydrocoral Millepora dichotoma. Symbiosis 4:171-184.
- Shagin, D., E. Barsova, Y. Yanushevich, A. Fradkov, K. Lukyanov, Y. Labas, T. Semenova, J. Ugalde, A. Meyers, J. Nunez, E. Widder, S. Lukyanov and M. Matz, 2004. GFP-like proteins as ubiquitous metazoan superfamily: Evolution of functional features and structural complexity. Mol. Biol. Evol., 21(5):841-850.
- Shibata, K., 1969. Pigments and a UV-absorbing substance in corals and a blue-green alga living in the Great Barrier Reef. Plant and Cell Physiol., 10: 325-335.
- Shimomura, O., F. Johnson and Y. Saiga, 1962. J. Cell Comp. Physiol. 59:223-239.
- Shkrob, M., Y. Yanushevich, D. Chudakov, N. Gurskaya, Y. Labas, S. Poponov, N. Mudrik, S. Lukyanov and K. Lukyanov, 2005. Far-red fluorescent proteins evolved from a blue chromoprotein from Actinia equina. Biochem. J., On-line publication.
- Smith, C., 2005. Spectral and microspatial effects of altering light intensity on fluorescent proteins of Porites furcata: A hard coral from the tropical western Atlantic. PhD dissertation, George Mason University. 214 pp.
- Sole-Cava, L.M., and J.P. Thorpe, 1992. Genetic divergence between the color morphs in populations of the common intertidal sea anemones Actinia equina and A. prasina (Anthozoa: Actiniaria) in the Isle of Man. Mar. Biol. 112:243-252.
- Sprung, J. and J. Delbeek, 1997. The Reef Aquarium – A Comprehensive Guide to the Identification and Care of Tropical Marine Invertebrates, Vol. 2. Ricordea Publishing, Coconut Grove, Florida. 546 pp.
- Stiel, A., S. Trowitzsch, G. Weber, M. Andresen, C. Eggeling, S. Hell, S. Jakobs and M. Wahl, 2007. 1.8-a bright state structure of the
- reversibly switchable fluorescent protein Dronpa guides the generation of fast-switching variants. Biochem. J., 402(1):35-42.
- Stephanenko, O., V. Verkhusha, V. Kazakov, M. Shavlovsky, I. Kuznetsova, V. Uversky and K. Turoverov, 2004. Comparative studies on the structure and stability of fluorescent proteins EGFP, zFP506, mRPF1, “dimer2”, and DsRed. Biochem., 43: 14913-14923.
- Sun, Y., E. Castner, Jr., C. Lawson and P. Falkowski, 2004. Biophysical characterization of natural and mutant fluorescent proteins cloned from zooxanthellate corals. FEBS Lett., 570(1-3):175-183.
- Takabayashi, M. and O. Hoegh-Guldberg, 1995. Ecological and physiological differences between the two colour morphs of the coral Pocillopora damicornis. Mar. Biol., 123: 705-714.
- Takabayashi, M., D. Carter, J. Lopez and O. Hoegh-Guldberg, 2003. Genetic variation of the scleractinian coral Stylophora pistillata, from western Pacific reefs. Coral Reefs 22(1): 17-22.
- Terskikh, A., A. Fradkov, A. Zaraisky, A. Kajava and B. Angres, 2002. Analysis of red mutants. Space around the fluorophore accelerates fluorescence development. J. Biol. Chem., 277(10):7633-7636.
- Todd, P., R. Sidle and L. Chou, 2002. Plastic corals from Singapore: 1. Coral Reefs, 21(4):391.
- Todd, P., R. Sidle and L. Chou, 2002a. Plastic corals from Singapore: 2. Coral Reefs, 21(4):407.
- Todd, P., R. Sidle and L. Chou, 2003. Erratum: Plastic corals from Singapore: 1. Coral Reefs, 22(3):306.
- Tsien, R., 1998. The green fluorescent protein. Annu. Rev. Biochem., 67:509-544.
- Tsutsui, H., S. Karasawa, H. Shimizu, N. Nukina and A. Miyawaki, 2005. Semi-rational engineering of a coral fluorescent protein into an efficient highlighter. EMBO Reports. 6:233-238.
- Tu, H., Q. Xiong, S. Zhen, X. Zhong, L. Peng, H. Chen, X. Jiang, W. Liu, W. Yang, J. Wei, M. Dong, W. Wu and A. Xu, 2003. A naturally enhanced green fluorescent protein from magnificent sea anemone (Heteractis magnifica) and its functional analysis. Biochem. Biophys. Res. Commun., 301(4):879-885.
- Turcic, K., A. Pettikiriarachchi, J. Battad, D. Wilmann, J. Rossjohn, S. Dove, R. Devenish and M. Prescott, 2006. Amino acid substitutions around the chromophore of Rtms5 influence polypeptide cleavage. Biochem. Biophys. Res. Comm., 340(4): 1139-1143.
- Tyree, S., 1998. Reef Building Stony Corals: The Natural Physical Environment. A Review of the Research Papers. D.E. Publishing, Murrieta, Ca. 418 pp.
- Ugalde, J., B. Chang and M. Matz, 2004. Evolution of coral pigments recreated. Science, 305(5689), 1433.
- Verkhusha, V., M. Matz, T. Sakurai and K. Lukyanov, 2003. GFP-like fluorescent proteins and chromoproteins of the class Anthozoa. In: Protein Structures: Kaleidoscope of Structural Properties and Functions. (V. Uversky, ed.) 405-439. Research Signpost Publishers, India.
- Verkhusha, V. and K. Lukyanov, 2004. The molecular properties and applications of Anthozoa fluorescent proteins and chromoproteins. Nature Biotechnology, 22(3):289-296.
- Vekhusha, V., D. Chudakov, N. Gurskaya, S. Lukyanov and K. Lukyanov, 2004. Common pathway for the red chromophore formation in fluorescent proteins and chromoproteins. Chem. Biol., 11:845-854.
- Vermeij, M., L. Delvoye, G. Nieuwland and R. Bak, 2002. Patterns in fluorescence over a Caribbean reef slope: The coral genus Madracis. Photosynthetica, 40(3): 423-429.
- Veron, J., 1993. Corals of Australia and the Indo-Pacific. University of Hawaii Press, Honolulu. 644 pp.
- Veron, J., 1995. Corals In Space and Time – The Biogeography and Evolution of the Scleractinia. Cornell University Press, Ithaca, NY. 321 pp.
- Veron, J., 2000. Corals of the World. Australian Institute of Marine Science and CCR Old Pty Ltd, Townsville. 1281 pp.
- Vestheim, H. and S. Kaartvedt, 2006. Plasticity in coloration as an antipredator strategy among zooplankton. Limnol. Oceanogr., 5(14): 1931-1934.
- Wachter, R. and S. Remington, 1999. Sensitivity of the yellow variant of GFP to halides and nitrate. Curr. Biol., 9(17); R627-R630.
- Weber, W. and H. Gras, 1980. Ultrastructure observations on changes in cell shapes in chromatophores of the Sea Urchin Centrostephanus longispinus. Cell Tiss. Res., 206:21-33.
- Weber, W. and M. Daumbach, 1974. Light-sensitivity of isolated pigment cells of the Sea Urchin Centrostephanus longispinus. Cell Tiss. Res., 148:437-440.
- Wiedenmann, J., C. Elke, K-D. Spindler, and W. Funke, 2000. Cracks in the beta-can: Fluorescent proteins from Anemonia sulcata (Anthozoa, Actinaria). Proc. Natl. Acad. Sci. USA, 97(26): 14091-14096.
- Wiedenmann, J., A. Schenk, C. Röcker, A. Girod, K-D. Spindler and G. Nienhaus, 2002. A far-red fluorescent protein with fast maturation and reduced oligomerization tendency from Entacmaea quadricolor (Anthozoa, Actinaria). Proc. Natl. Acad. Sci. USA, 99, 18: 11646-11651.
- Wiedenmann, J., A. Schenk, C. Röcker, A. Girod, K-D. Spindler and G. Nienhaus, 2002a. Correction for: A far-red fluorescent protein with fast maturation and reduced oligomerization tendency from Entacmaea quadricolor (Anthozoa, Actinaria). Proc. Natl. Acad. Sci. USA, 99, 20:14091-14096.
- Wiedenmann, J., S. Ivanchenko, F. Oswald, F. Schmitt, C. Röcker, A. Salih, K-D. Splinder and G. Nienhaus, 2004. EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion. Proc. Natl. Acad. Sci. USA, 101(45):15905-15910.
- Wiedenmann, J., S. Ivanchenko, F. Oswald, and G. Nienhaus, 2004. Mar. Biotech., 6(3):
- Wilmann, P., J. Battad, T. Beddoe, S. Olsen, S. Smith, S. Dove, R. Devenish, J. Rossjohn and M. Prescott, 2005. The 2.0 Å crystal structure of a pocilloporan at pH 3.5: The structural basis for the linkage between color transition and halide binding. Photochem. Photobiol., 82(2):359-66.
- Yampolsky, I., S. Remington, V. Martynov, V. Potapov, S. Lukyanov and K. Lukyanov, 2005. Synthesis and properties of the chromophore of the asFP595 chromoprotein from Anemonia sulcata. Biochem., 44(15):5788-5793.
- Yanushevic, Y., M. Bulina, N. Gurskaya, A. Savitskii and K. Lukyanov, 2002. Key amino acid residues responsible for the color of green and yellow fluorescent proteins from the coral polyp Zoanthus sp. Russ. J. Bioorg. Chem., 28(4): 274-277.
- Yentsch, C. and D. Phinney. CoBOP Coral Reefs: Optical closure of a coral reef submarine light field. On-line publication.
- Yu, J-K, T-H Liao, C. Chen, T-H Liao, L-S Fang and W-S Tsai, 2000. Do color patterns of Pocillopora damicornis reflect zooxanthellae diversity? Coral Reefs, 19(1): 98-99.
- Yu, M-H., A. Glazer, K. Spencer and J. West, 1981. Phycoerythrins of the red alga Callithamnion. Plant Physiol., 68:482-488.
- Zagranichny, V., N. Rudenko, A. Gorokhovatsky, M. Zakharov, Z. Shenkarev, T. Balashova and A. Arseniev, 2004. zFP538, a yellow fluorescent protein from coral, belongs to the DsRed subfamily of GFP-like proteins but possesses the unexpected site of fragmentation. Biochem, 43(16):4764-4672.
- Zawada, D. and J. Jaffe, 2003. Changes in the fluorescence of the Caribbean coral Montastraea faveolata during heat-induced bleaching. Limnol. Oceanogr., 481(1, part 2):412-425.
- Zimmer, M., 2005. Glowing Genes: A Revolution in Biotechnology. Prometheus Books, Amherst, New York. 222 pp.



0 Comments