Science is a journey full of surprises and the story behind the current interest in coral fluorescence and pigmentation is no less full of twists and turns. This tale is one of the reef-keeping hobby in Russia and Japan, a couple of fortuitous laboratory accidents, a revolution in biomedical research, revelations and fortunes made, discovery of a new symbiosis, as well as the small contributions of reef hobbyists from other parts of the globe. While coral coloration is today less likely to be the subject of intense interest (and debate) within the hobby than it was just 5 years ago, the situation is much different in peer-reviewed literature, with researchers producing a staggering amount of information on marine invertebrate fluorescent and non-fluorescent pigments. It is estimated that 26,000 papers mentioning fluorescent proteins will be in print by the end of 2006. Not all of these – few, in fact – are of much interest to the advanced aquarist, however, some offer glimpses of those conditions required to activate, or inactivate, the vivid coloration we sometimes see in our captive reefs. Of course, explaining pretty colors in an aquarium (or reef) is not usually researchers’ main goals. The revolutionary use of certain proteins as molecular markers (or ‘highlighters’) in the biomedical field has driven the identification, refinement and genetic modification of many ‘wild type’ fluorescent proteins. Other researchers have studied coral pigments in order to remotely sense (by aircraft or satellite) the light reflected and fluoresced by natural reefs. In this manner, vast tracts of reefs can be examined in relatively little time and an assessment of reef health can be made.
Why should reef hobbyists concern themselves with fluorescence? Isn’t it generally accepted that light energy is a prime requirement for expression of coloration? What if could understand and anticipate responses of a pigment to different visible light bandwidths, ultraviolet radiation or other stimuli? What of the effect of nutrients and supplement additions (such as iodine)? Not all pigments are fluorescent but all are selective in the wavelengths they absorb or reflect. With the recent introduction of relatively inexpensive spectrometers (such as those from Ocean Optics or Edmund Optics) more hobbyists can gain access to these powerful tools (for a review of the Ocean Optics spectrometer, see Riddle, 2006). An understanding of fluorescent proteins (at least their excitation and emission peaks – see definitions below) is essential for understanding the apparent color of corals, since fluorescence can influence the color perceived by the observer. Further, understanding of fluorescence and its effects is essential when we examine reflected light later in this series. Realizing the potential reasons for color transitions of our captive corals might enable us to see these shifts as environmental indicators, good or bad. But, perhaps most importantly to us as hobbyists, manipulating the artificial conditions with aquaria might allow us to alter pigmentation within certain invertebrates and create animals with ‘customized’ coloration.
This modest series of articles will review some recent conclusions drawn from experiments from the world’s leading authorities on fluorescent and non-fluorescent pigments. When possible, recommendations of areas for further investigation by hobbyists will be suggested. Observations by hobbyists can be of great use. It is difficult to ignore mountains of anecdotal evidence – it has been generally accepted within the hobby that light is sometimes a critical factor in promotion of some coral coloration.
In 1995, hobbyist (and scientist) Craig Bingman was perhaps the first to recognize that the rainbow of colors in marine invertebrates shared a common ancestor in a green fluorescent protein; an addendum followed in 1999. All observations have not proven prophetic – it was once very popular within the hobby to associate all coral coloration with pigments found within zooxanthellae. While it is true that light absorbed by the photopigments of symbionts does play an important part in the overall coloration perceived by the human eye, it is generally a mistake to ascribe fluorescence to chlorophyll and a definite error to link it to peridinin, beta-carotene and some other photopigments (the role these pigments play in determining the apparent coral color will be examined in a separate article. Phycoerythrin is a notable exception as it can lend an orange fluorescent color to corals under proper conditions). Articles that state differently-colored zooxanthellae are responsible for fluorescence are misleading. In the same vein, coloration was wrongly attributed to natural sunscreens that absorb and mitigate the effects of damaging UV radiation. By extension, the idea was formed within the hobby that ultraviolet energy is necessary for colorful corals (we know now that these sunscreens – MAAs for mycosporine-like amino acids – are colorless and do not lend color. We’ll examine in this series the recent evidence for the necessity of UV in color shifts and relate this to aquarium husbandry practices).
Peer-reviewed publications have not always been correct either. For instance, Kennedy (1979) described colorful invertebrate pigments as porphyrins (naturally abundant in plant and animal tissues. Porphyrin is a Greek word meaning ‘purple’ – they are chromatic pigments). Kennedy reviewed early work by Moseley (1877) which describes the red pigment found in Discosoma, Actinia, Flabellum, Stephanophyllia and Fungia as ‘polyperythrin’ with excitation wavelengths in the yellow-green portion of the spectrum.
Red fluorescence found in stony corals Montastraea cavernosa and Mussa angulosa tempted Kennedy to describe them as porphyrins as well. Even with missteps the pieces of the coloration puzzle are slowly but surely falling into place.
For general interest articles examining coral fluorescence in aquaria, see works of Riddle and Amussen (1998), Delbeek (2003), Calfo (2005), Blundell (2005), Finét and Lesage (2005) and Credabel (2006). Interesting comments concerning a few specific fluorescent pigments can be found in Delbeek and Sprung, 2005, as well as in Tyree (1998). Charles Mazel maintains a fascinating website on marine fluorescence at www.nightsea.com.
My fascination with coral pigments began when I saw a blue-tipped Acropora (formosa?) specimen. This was in the early 1990’s, and many hobbyists (including myself) were of the opinion that it was practically impossible to artificially over-illuminate an aquarium containing stony corals such as Acropora. So, I was very curious why a coral would reflect light energy necessary for photosynthesis (the coloration did not appear to be fluorescent). I did not know it at the time, but this simple observation would be a turning point in my life. I’m still unsure of the reason why that Acropora fragment reflected blue wavelengths, but my fascination would become a passion in the quest for answers about coelenterate coloration.
The first in this series will present information on a few of the fluorescent proteins found in marine invertebrates. Generally, fluorescent proteins are best viewed under relatively narrow bandwidths, such as those produced by actinic and black lights. However the mere categorization of ‘fluorescent’ and ‘non-fluorescent’ proteins presents difficulties – a protein can be fluorescent in a technical sense but the ‘glow’ may be of low intensity and invisible to the unaided eye. In addition, environmental conditions (such as light quality and/or quantity or chemical changes) may trigger a mutation of a non-fluorescent protein to a fluorescent species or vice versa. This leads us to a point where some definitions are in order before we continue.
Fluorescence. Fluorescence is a phenomenon in which a material absorbs light of one color (wavelength) and emits it at a different color (wavelength). Absorption occurs when an incoming photon (light particle) causes an electron to move from a stable ground state to a higher energy, unstable excited state. One of the ways for the excited electron to return to the ground state is to ‘jump’ back down, emitting a photon of light. There is always some energy lost to heat in the process, so the emitted photon has less energy than the original photon. The energy of a photon is related to its wavelength, which we perceive as color – higher energy corresponds to shorter wavelengths, lower energy to longer wavelengths. This shift in color was noted by the brilliant, if slightly off-beat, Sir George Stokes, who first described fluorescence in 1852.
See this informative web site for further information.
Emission spectrum. The emission spectrum is a measurement of the emitted energy as a function of wavelength. It is generally presented as a graph (see Figure 1). The spectrum will have a maximum but may also have secondary peaks, called ‘shoulders’. In text the spectrum is often described by the wavelength of the emission peak. Some emission spectra cover a broad range of wavelengths, while others are quite sharp. This is described by the Full Width at Half Maximum (FWHM), the spectral width in nanometers at the level that is 50% of the value at the peak. The emission spectrum can be measured with a basic spectrometer like the units from Ocean Optics.
Excitation spectrum. The excitation spectrum is a measurement of the relative ability of different wavelengths of light to stimulate (excite) the fluorescence (see Figure 1). Like the emission spectrum, the excitation spectrum will have a maximum but may also have secondary peaks. The excitation spectrum tells you what wavelengths of light will be good at making that particular substance fluoresce. Unlike the emission spectrum, the excitation spectrum is difficult to measure without specialized, expensive instrumentation. To make the measurement you have to vary the wavelength of the incident light in very fine increments and measure the fluorescence response at each one.
Fluorescence lifetime. Fluorescence is short lived – once the excitation wavelengths are absent, fluorescence decays in a fraction of a second (billionths of a second), and the phenomenon ends.
Stokes shift. The Stokes shift describes the difference (in nanometers – nm) between the maximum excitation and maximum emission wavelengths. Stokes shifts of coral pigments can range from just a few nanometers to ~180nm and perhaps more.
Quantum Yield. The ratio of photons emitted through fluorescence to photons absorbed by a pigment is called the Fluorescence Quantum Yield. The higher the fluorescent quantum yield, the more efficient a pigment is at fluorescing absorbed light. Quantum yields and relative brightness vary significantly among reports, and could be due to many factors including protein purity and concentration, maturation conditions, protein source and so on (Terskikh et al., 2002).
Relative Brightness. Relative brightness is the comparison of emitted fluorescence to that of a standard (such as the fluorescent pigment from the jellyfish Aequorea, or a fluorescent dye). If the pigment’s ‘glow’ is less intense than that of the reference, the relative brightness is less <1. It is possible for the relative brightness to exceed that of the standard in which case, of course, the relative brightness will exceed 1.
Fluorescence Coupling and Förster Resonance Energy Transfer (FRET). Fluorescence Coupling occurs when a pigment’s emission overlaps the excitation spectrum of another pigment and energy is transferred between them via a radiative mechanism (that is, a photon is emitted from one pigment and absorbed by the other – this is a very low probability event). In this manner, it is possible that sequential transformation of wavelengths could occur. Coupling is possible between coral fluorescent pigments (FPs). Förster Resonance Energy Transfer (FRET, sometimes called Fluorescent Resonance Energy Transfer) is a non-radiative, ‘vibrational’ energy transfer between intimate donor and acceptor pigments. FRET is maximal when the distance between pigment granules in corals is <10µm (Salih et al., 2000). When it does occur in nature, energy transfer by FRET can be very efficient.

Figure 1. An idealized chart demonstrating the concepts of fluorescence excitation and emission spectra, as well as the Stokes shift.
Photoconversion. Photoconversion is a change in absorption and emission properties of a fluorescent protein caused by incident light energy. Ultraviolet, violet, blue and green bandwidths are known to cause photoconversions. At least two sorts of photoconversion are known to occur in anthozoan pigments. These are known as ‘reddening’ and ‘photobleaching.’ Generally ‘reddening’ describes a process by which green fluorescent compounds are converted to those exhibiting red fluorescence.
Photobleaching usually describes a change from green fluorescence to no fluorescence at all. Photoconversion is not responsible for all ‘reddening’. Some color shifts re due to:
Chemical oxidation. Oxygen plays a role in the maturation (colorless to green color shift) in the green fluorescent protein (GFP) of Aequorea victoria. It also plays an essential part in ‘reddening’ of fluorescent proteins in the false coral Discosoma, and ‘greening’ of a purple-red chromoprotein found in Goniopora tenuidens. We’ll discuss photoconversion in much more detail later in this series.
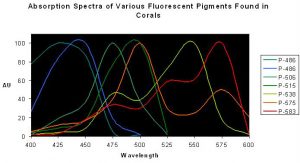
Figure 2. Normalized excitation spectra of various fluorescent pigments found in anthozoans. Note the distinct double or triple ‘humps’ of Pigments 538, 575 and 583 – the lesser peaks are called ‘shoulders’. Spectra from various references.
Photoactivatable Fluorescent Proteins (PAFPs). Some proteins of interest to aquarists can convert from a non-fluorescent state to one of fluorescence given the proper environmental triggers. Visible light (of various wavelengths and intensities) are required for photoactivation of these ‘chameleons’. Ultraviolet radiation can also activate certain pigments (more on this subject later in this series – don’t pull those UV shields yet!).
Pigment Characterization
Researchers have variously reported fluorescent proteins by color groupings (cyan-green-yellow-red), clade, numerical emission wavelength, or as overt and covert fluorescence. Fluorescence can play a major role in the appearance of an organism (such as a coral illuminated by a black light or spectrum weighted in the appropriate excitation wavelengths as found in ‘actinic’ fluorescent lamps or high-Kelvin metal halide lamps). Fluorescence can also play a part in the perceived color of a coral in daylight – strong fluorescence can ‘mix’ with the light reflected from a coral (This mixing of fluorescence and reflected light will be examined in a separate article). Not all coral pigments are fluorescent. These definitions will be used in this series of articles:
Chromoproteins. For our purposes, chromoproteins will describe the set of anthozoan pigments that do not visibly fluoresce (if at all). This includes the non-fluorescent pink and blue coral pigments (collectively called ‘pocilloporans’, Dove et al., 1995) and purple/red pigments found in some corals and anemones. ‘CP’ is the abbreviation for chromoprotein.
Fluorescent proteins can be subdivided into two categories:
Photopigments are organic compounds that harvest light energy for use in photosynthesis. Also called ‘accessory’ or ‘antennae’ pigments, the fluorescent substances of most interest to us in the discussion of coral fluorescence include chlorophyll a and phycoerythrin.
Green Fluorescent Proteins and homologues. Green fluorescent proteins (GFPs) and GFP-like proteins are those fluorescent pigments not intimately associated with photosynthesis (coral fluorescence is not due to the color of zooxanthellae!). Fluorescent proteins are variously colored cyan (CyFP), green (GFP), yellow (YFP) or red (RFP). These may have some role in photoprotection of the coral host and/or its symbiotic zooxanthellae. There are arguments for and against this line of reasoning. This will be discussed later in this series of articles.
‘Kindling’ Proteins (KPs) are sometimes referred to as Kindling Fluorescent Proteins (KFPs) and do not fall neatly into a fluorescent/non-fluorescent category as they can be transiently fluorescent. The transformation of a non-fluorescent protein to one capable of fluorescence is known as ‘kindling.’ Light (quality- often green wavelengths – and quantity or intensity) is often the environment trigger for kindling. See this site for an interesting tutorial:

Figure 3. Though gaudily colored, the purple coloration of this Acropora specimen is due to the presence of a chromoprotein, and is not visibly fluorescent. The coral does, however, contain a fluorescent pigment. See Figure 4.
Categorization of Coral Pigments
Various texts categorize visible radiation into divisions of perceived color and associated wavelengths. These categorizations do not always agree. I have unilaterally chosen a set of colors and associated wavelengths from one of my fossilized college text books and will list pigments accordingly (these are my articles – I can do anything I want!).
There is yet one more definition – the method of determining the shorthand for pigments. Again, there is no universal agreement. Matz et al., 1999 and Labas et al., 2002 use the genus name followed by a pigment number (I assume this is the order in which the pigments were identified, such as Zoanthus 1 or Discosoma 3). Matz et al. (1999) list two pigments from Zoanthus, and common sense dictates that we call them Zoanthus 1 and Zoanthus 2, yet Labas et al. (2002) list a pigment called Zoanthus 2 that has a different excitation and emission than either of the pigments listed in the Matz paper. There must, hopefully, be a better method. Some papers used abbreviations such as ‘mcavFP580’ (‘mcav’ means the pigment was isolated from the stony coral Montastraea cavernosa, while ‘FP’ stands for ‘fluorescent protein’ and the numbers describes the wavelength of the maximum fluorescent emission). This is perhaps unwieldy for our purposes, and I for several reasons have chosen to use the early (and shorter) abbreviations such as P-575 (‘P’ is for ‘pigment’ and ‘575’ is the wavelength of maximum fluorescence emission). There may be several coral genera containing a pigment with the same fluorescent emission, and the same pigment may exhibit small shifts in its exact peak location. The answer seems to list each pigment, by emission, and list the host animal in a separate column.

Figure 4. A fragment of the coral pictured in Figure 3, but viewed under a black light emitting a UV-A radiation at a maximum of 365nm. This green fluorescent pigment hides the normal purple coloration of the animal. This Acropora specimen has at least one fluorescent pigment and at least one non-fluorescent chromoprotein.
For non-fluorescent pigments (chromoproteins, or CPs) the wavelength number stands for the maximum absorption. Bear in mind that it is entirely possible for a single specimen to contain multiple pigments (whether they are fluorescent, or not). See Figures 3 and 4.
It is an important concept but most hobbyists are well aware of this fact – this phenomenon is apparent when specimens are viewed under ‘actinic’ fluorescent lamps.
Fluorescent and reflective pigments found in coral tissues are generally water-soluble. Loss of pigments in nature or in an aquarium would be expected to be quite low; however, corals contribute fluorescent compounds to the water column (Gentien, 1981). This scientist demonstrated that amounts of fluorescent compounds increased in prefiltered seawater when Acropora formosa, A. cerealis, A. hyacinthus, Stylophora pistillata, Favia favus, Favites flexuosa and Leptoria phyrgia specimens were incubated.
Koh (1997) found that the non-photosynthetic coral Tubastraea faulkneri secreted bioactive compounds to the water column, and some of these compounds could be visualized through fluorescence on developed thin-layer chromatographs. Allelopathic intra-actions and interactions of coral color morphs will be discussed briefly later in this series.

Figure 5. One interpretation of anthozoan colors’ phylogenetic tree (After Shagin et al., 2004). Clade ‘A’ GFP (found in some anemones) will fluoresce bright green during its transition from green to red. Clade B GFP demonstrates far less fluorescence than Clade ‘A’ during transition. Clade C GFPs from zoanthids are like Clade ‘A’ in that they strongly fluoresce. Clade D (mostly in stony corals) requires ultraviolet radiation or violet/blue light to mature (Labas, 2002). Note: Mazel, 1995, describes a yellow fluorescent protein in the scleractinian Agaricia.
Phylogenetic Classifications
Though still in its infancy, computer software can sort (for our purposes) zooxanthellae and even pigments into ‘clades.’ At present, at least four pigment clades are known (A-D), which contain differing variations of cyan, green, yellow and red fluorescent proteins plus non-fluorescent chromoproteins. Figure 5 is one interpretation of pigments’ phylogenetic tree.
Software of this type is considered by some as subject to fault, but it is an interesting concept.
A Listing of Fluorescence Proteins
Table 1 is a compilation of over 90 fluorescent proteins found in various invertebrate species (non-fluorescent chromoproteins will be listed under separate cover). I have color-coded the appropriate fields to indicate approximate color of the wavelength listed. The column on the left lists the pigment’s name. Next is the maximum emission column followed by two additional columns listing second and third emissions (shoulders), if present. The excitation columns follow. (Note: Observed excitation and emission spectra can vary between instruments). Finally, the last two columns list the invertebrate host (in alphabetical order) and then a reference listing for those interested in further reading. I have used the genus and species identifications listed within various papers. Coral taxonomy is in a constant state of flux and I am not (nor do I have the time to be) qualified to revise identifications. This listing is as complete as I can make it with the references available to me. A fluorescent emission peaking at a certain wavelength does not necessarily mean that the animal host will appear the color that the wavelength indicates (individual coral colonies can contain ‘over 10,’ and possibly more, fluorescent pigments, Salih et al., 2004). Perceived color is a result of many factors.
| Pigment | Emission | 2 | 3 | Excitation | 2 | 3 | Found in: | Reference |
|---|---|---|---|---|---|---|---|---|
| P-445 | 445 | * | * | 340 | * | * | Acropora aspera | Dove et al., 2001 |
| P-490 | 490 | 514 | * | 480 | 501 | * | Acropora aspera | Papina et al., 2002 |
| P-500 | 500 | * | * | 480 | * | * | Acropora aspera | Dove et al., 2001 |
| P-514 | 514 | 490 | * | 501 | 480 | * | Acropora aspera | Papina et al., 2002 |
| P-630 | 630 | * | * | 576 | * | * | Acropora aspera | Dove et al., 2001 |
| P-480 | 480 | 510 | * | 481 | * | * | Acropora aspera (green band)* | Papina et al., 2002 |
| P-476 | 476 | 510 | 575 | 500 | 475 | * | Acropora aspera (orange band I)* | Papina et al., 2002 |
| P-478 | 478 | 510 | 575 | 501 | 475 | * | Acropora aspera (orange band II)* | Papina et al., 2002 |
| P-487 | 487 | 517 | * | * | * | * | Acropora cervicornis | Mazel,1995 |
| P-518 | 518 | * | * | * | * | * | Acropora cytheria @ Waikiki Aquarium | Hochberg et al., 2004 |
| P-490 | 490 | * | * | 425 | * | * | Acropora digitifera | Dove etal., 2001 |
| P-495 | 495 | * | * | * | * | * | Acropora digitifera | Gilmore et al., 2003 |
| P-518 | 518 | * | * | * | * | * | Acropora digitifera | Hochberg et al., 2004 |
| P-590 | 590 | * | * | 570 | * | * | Acropora digitifera | Dove et al., 2001 |
| P-485 | 485 | * | * | 420 | * | * | Acropora horrida | Dove et al., 2001 |
| P-400 | 400 | * | * | 345 | * | * | Acropora horrida | Dove et al., 2001 |
| P-625 | 625 | * | * | 575 | * | * | Acropora horrida | Dove et al., 2001 |
| P-490 | 490 | * | * | 405 | * | * | Acropora millepora | Cox and Salih, 2005 |
| P-504 | 504 | * | * | 405 | * | * | Acropora millepora | Cox and Salih, 2005 |
| P-593 | 593 | * | * | 405** | * | * | Acropora millepora | Cox and Salih, 2005 |
| P-409 | 409 | * | * | * | * | * | Acropora nastua | Gilmore et al., 2003 |
| P-482 | 482 | 515 | * | 451 | 430 | * | Acropora nastua | Papina et al., 2002 |
| P-483 | 483 | * | * | 427 | 451 | * | Acropora nastua (green band)* | Papina et al., 2002 |
| P-486 | 486 | * | * | 384 | * | * | Acropora nobilis | Salih et al., 2000 |
| P-484 | 484 | 515 | * | 450 | 502 | * | Acropora secale | Papina et al., 2002 |
| P-482 | 482 | 515 | * | 452 | 500 | 425 | Acropora secale (green band)* | Papina et al., 2002 |
| P-495 | 495 | * | * | 472 | * | * | Acropora sp. | Karasawa et al., 2003 |
| P-485 | 485 | 517 | 555 | 465 | 505 | * | Acropora tenuis | Papina et al., 2002 |
| P-517 | 517 | 555 | 485 | 505 | 465 | * | Acropora tenuis | Papina et al., 2002 |
| P-480 | 480 | 515 | * | 470 | 504 | * | Acropora tenuis (green band)* | Papina et al., 2002 |
| P-509 | 509 | * | * | 397 | * | * | Aequoria victoria | Tsien, 1998 |
| P-508-620 | 508-620 | * | * | * | * | * | Agaricia agaricites @ 60m | Vermeij et al., 2002 |
| P-565 | 565 | * | * | 490 | * | * | Agaricia humilis | Mazel et al., 2003 |
| P-486 | 486 | * | * | 426 | * | * | Agaricia sp. | Mazel, 1997 |
| P-497 | 497 | 527 | * | * | * | * | Agaricia sp. | Mazel, 1995 |
| P-513 | 513 | 545 | 490 | * | * | * | Agaricia sp. | Mazel, 1995 |
| P-515 | 515 | * | * | * | * | * | Agaricia sp. | Mazel, 1995 |
| P-557 | 557 | 600 | 545 | * | * | * | Agaricia sp. | Mazel, 1995 |
| P-542 | 542 | * | * | * | * | * | Agaricia undata @ 40m | Vermeij et al., 2002 |
| P-486 | 486 | * | * | 458 | * | * | Anemonia majano | Matz et al., 1999 |
| P-499 | 499 | * | * | 480 | 403 | 278 | Anemonia sculata | Wiedenmann et al., 2002 |
| P-595 | 595 | * | * | 574 | * | * | Anemonia sculata | Wiedenmann et al., 2000 |
| P-522 | 522 | * | * | 499 | ** | * | Anemonia sculata var. rufescens | Wiedenmann et al., 2000 |
| CP-562 | * | * | * | 562 | * | * | Anemonia sulcata, immature P-595 | Wiedenmann et al., 2002 |
| P-565 | 565 | * | * | 548 | * | * | Cerianthus sp. | Ip et al., 2004 |
| P-685 | 685 | * | * | Violet/ Blue | Red | * | Chlorophyll – common to healthy hermatypic corals | Multiple references |
| P-484 | 484 | * | * | 456 | * | * | Clavularia sp. | Matz et al., 1999 |
| P-515 | 515 | * | * | * | * | * | Colpophyllia natans | Fux and Mazel, unpublished |
| P-496 | 496 | * | * | 399 | 482 | * | Condylactis gigantea | Labas et al., 2002 |
| P-497 | 497 | 527 | * | * | * | * | Condylactis gigantea | Mazel, 1995 |
| P-508 | 508 | * | * | 494 | * | * | Dendronephthya | Labas et al., 2002 |
| P-575 | 575 | * | * | 557 | * | * | Dendronephthya | Pakhomov et al., 2004 |
| P-486 | 486 | * | * | ~448 | * | * | Diploria labyrinthiformis | Fux and Mazel, unpublished |
| P-500 | 500 | * | * | 475 | * | * | Discosoma sp. | Gross et al., 2000 |
| P-583 | 583 | * | * | 558 | 530 | 487 | Discosoma sp. | Matz et al., 1999; Baird et al., 2000 |
| P-593 | 593 | * | * | 558 | * | * | Discosoma sp. 2 | Fradkov et al., 2000 |
| P-512 | 512 | * | * | 503 | * | * | Discosoma sp. 3 | Labas et al., 2002 |
| P-593 | 593 | * | * | 573 | * | * | Discosoma sp. 3 | Labas et al., 2002 |
| P-483 | 483 | * | * | 443 | * | * | Discosoma striata | Matz et al., 1999 |
| P-611 | 611 | * | * | 559 | * | * | Entacmaea quadricolor | Wiedenmann et al., 2002 |
| P-487 | 487 | 517 | * | * | * | * | Eusmilia fastigata | Mazel, 1995 |
| P-518 | 518 | * | * | 503 | * | * | Family Pectiniidae | Ando et al., 2004 |
| P-517 | 517 | * | * | 507 | * | * | Favia favus | Tsutsui et al., 2005 |
| P-593 | 593 | * | * | 583 | * | * | Favia favus | Tsutsui et al., 2005 |
| P-507 | 507 | 536 | 575 | * | * | * | Favia fragum | Mazel, 1995 |
| P-561 | 561 | * | * | 548 | * | * | Fungia concinna | Karasawa et al., 2004 |
| P-505 | 505 | * | * | 492 | * | * | Galaxea fascicularis | Karasawa et al., 2003 |
| P-480 | 480 | * | * | * | * | * | Goniopora tenuidens | Salih et al., 2004 |
| P-494 | 494 | * | * | * | * | * | Goniopora tenuidens | Salih et al., 2004 |
| P-509 | 509 | * | * | * | * | * | Goniopora tenuidens | Salih et al., 2004 |
| P-520 | 520 | * | * | 488 | * | * | Goniopora tenuidens | Salih et al., 1999 |
| P-582 | 582 | * | * | * | * | * | Goniopora tenuidens | Salih et al., 2004 |
| P-500 | 500 | * | * | 405 | 481 | * | Heteractis crispia | Labas et al., 2002 |
| P-510 | 510 | * | * | 490 | 420 | * | Heteractis magnifica | Tu et al., 2003 |
| P-446(?) | 446 | * | * | 380 | * | * | Leptoseris fragilis | Schlichter et al., 1985 |
| P-516 | 516 | * | * | * | * | * | Lobophyllia hemprichii | Nienhaus et al., 2005 |
| P-483 | 483 | * | * | 572 | 454 | 510 | Lobophyllia hemprichii (red) | Salih et al., 2004 |
| P-515 | 515 | * | * | * | * | * | Lobophyllia hemprichii (red) | Salih et al., 2004 |
| P-519 | 519 | * | * | * | * | * | Lobophyllia hemprichii (red) | Salih et al., 2004 |
| P-580 | 580 | * | * | * | * | * | Lobophyllia hemprichii (red) | Salih et al., 2004 |
| P-581 | 581 | * | * | * | * | * | Lobophyllia hemprichii (red) | Salih et al., 2004 |
| P-519-557 | 519-557 | 590 | * | * | * | * | Madracis carmabi | Vermeij et al., 2002 |
| P-520-555 | 520-555 | 590 | * | * | * | * | Madracis carmabi | Vermeij et al., 2002 |
| P-522-623 | 522-623 | * | * | * | * | * | Madracis formosa @ 40m | Vermeij et al., 2002 |
| P-534 | 534 | 575 | * | * | * | * | Madracis formosa @ 60m | Vermeij et al., 2002 |
| P-532 | 532 | 590 | * | * | * | * | Madracis pharensis (brown tissue @ 10m) | Vermeij et al., 2002 |
| P-533 | 533 | 590 | * | * | * | * | Madracis pharensis (brown tissue @ 20m) | Vermeij et al., 2002 |
| P-535 | 535 | 587 | * | * | * | * | Madracis pharensis (brown tissue @ 60m) | Vermeij et al., 2002 |
| P-520-570 | 520-570 | 590 | * | * | * | * | Madracis pharensis (green tissue @ 10m) | Vermeij et al., 2002 |
| P-520-570 | 520-570 | 590 | * | * | * | * | Madracis pharensis (green tissue @ 20m) | Vermeij et al., 2002 |
| P-520-570 | 520-570 | 590-620 | * | * | * | * | Madracis pharensis (green tissue @ 40m) | Vermeij et al., 2002 |
| P-520-570 | 520-570 | 590 | * | * | * | * | Madracis pharensis (green tissue @ 60m) | Vermeij et al., 2002 |
| P-519-570 | 519-570 | 590-615 | * | * | * | * | Madracis pharensis (green tissue) | Vermeij et al., 2002 |
| P-537 | 537 | 590 | * | * | * | * | Madracis pharensis (grey and blue tissue @ 10m) | Vermeij et al., 2002 |
| P-532 | 532 | * | * | * | * | * | Madracis pharensis (red tissue @ 10m) | Vermeij et al., 2002 |
| P-587 | 587 | 609 | * | * | * | * | Madracis pharensis @ 40m (brown tissue) | Vermeij et al., 2002 |
| P-560 | 560 | 590 | * | * | * | * | Madracis pharensis @ 10m (grey tentacles) | Vermeij et al., 2002 |
| P-519-559 | 519-559 | 590 | * | * | * | * | Madracis senaria | Vermeij et al., 2002 |
| P-520-558 | 520-558 | 590 | * | * | * | * | Madracis senaria | Vermeij et al., 2002 |
| P-561 | 561 | 587-616 | * | * | * | * | Madracis senaria @ 40m | Vermeij et al., 2002 |
| P-559 | 559 | 590 | * | * | * | * | Madracis senaria @ 60m | Vermeij et al., 2002 |
| P-487 | 487 | 515 | 575 | * | * | * | Manicina areolata | Mazel, 1995 |
| P-487 | 487 | 515 | * | * | * | * | Meandrina meandrites | Mazel,1995 |
| P-480 | 480 | * | * | * | * | * | Montastraea annularis | Manica and Carter, 2000 |
| P-484 | 484 | 499 | * | * | * | * | Montastraea annularis | Mazel, 1995 |
| P-486 | 486 | * | * | * | * | * | Montastraea annularis | Mazel, 1997 |
| P-503 | 503 | 479 | 578 | * | * | * | Montastraea annularis | Mazel, 1995 |
| P-510 | 510 | 479 | * | * | * | * | Montastraea annularis | Mazel, 1995 |
| P-515 | 515 | * | * | 505 | * | * | Montastraea annularis | Mazel, 1997; Manica & Carter, 2000 |
| P-517 | 517 | 551 | 483 | * | * | * | Montastraea annularis | Mazel, 1995 |
| P-486 | 486 | * | * | 440 | * | * | Montastraea cavernosa | Lesser et al., 2000 |
| P-505 | 505 | * | * | * | * | * | Montastraea cavernosa | Kelmanson & Matz, 2003 |
| P-510 | 510 | * | * | 440 | * | * | Montastraea cavernosa | Mazel et al., 2003 |
| P-510-520 | 510-520 | * | * | 440 | * | * | Montastraea cavernosa | Lesser et al., 2000 |
| P-514 | 514 | * | * | * | * | * | Montastraea cavernosa | Kelmanson & Matz, 2003 |
| P-518 | 518 | * | * | * | * | * | Montastraea cavernosa | Kelmanson & Matz, 2003 |
| P-519 | 519 | * | * | ~505 | * | * | Montastraea cavernosa | Kelmanson & Matz, 2003 |
| P-522 | 522 | * | * | * | * | * | Montastraea cavernosa | Kelmanson & Matz, 2003 |
| P-575 | 575 | ~630 | * | ~525 | ~570 | * | Montastraea cavernosa | Mazel, 1997 |
| P-516 | 516 | * | * | 506 | ~480 | * | Montastraea cavernosa (=mc2/3/4 – see P-515) | Labas et al., 2002 |
| P-582 | 582 | 630 | * | 508 | 572 | * | Montastraea cavernosa (mc1) | Kelmanson & Matz, 2003 |
| P-515 | 515±3 | * | * | 505±3 | * | * | Montastraea cavernosa (mc2/3/4) | Kelmanson & Matz, 2003 |
| P-495 | 495 | * | * | 435 | * | * | Montastraea cavernosa (mc5) | Kelmanson & Matz, 2003 |
| P-507 | 507 | * | * | 495 | * | * | Montastraea cavernosa (mc6) | Kelmanson and Matz, 2003 |
| P-580 | 580 | 520 | * | 508 | 572 | * | Montastraea cavernosa (mcavRFP) | Labas et al., 2002 |
| P-510-623 | 510-623 | * | * | * | * | * | Montastraea cavernosa @ 40m | Vermeij et al., 2002 |
| P-534 | 534 | 593 | * | * | * | * | Montastraea cavernosa @ 60m | Vermeij et al., 2002 |
| P-486 | 486 | * | * | 440 | * | * | Montastraea faveolata | Lesser et al., 2000 |
| P-510-520 | 510-520 | * | * | 440 | * | * | Montastraea faveolata | Lesser et al., 2000 |
| P-575 | 575 | * | * | 506 | 555 | * | Montipora (digitata/angulata) | Mazel, unpublished |
| P-485 | 485 | * | * | 420 | 460 | 465 | Montipora caliculata | Dove et al., 2001 |
| P-490 | 490 | * | * | 420-450 | * | * | Montipora monasteriata | Dove et al., 2001 |
| P-610 | 610 | * | * | 570 | * | * | Montipora monasteriata | Dove et al., 2001 |
| P-620 | 620 | * | * | 440 | * | * | Montipora sp. | Kogure et al., 2006 |
| P-515 | 515 | * | * | * | * | * | Mycetophyllia lamarckiana | Mazel, 1997 |
| P-515 | 515 | * | * | ~494 | * | * | Mycetophyllia sp. | Fux and Mazel, unpublished |
| P-575 | 575 | * | * | ~569 | ~540 | ~500 | Phycoerythrin within symbiotic cyanobacteria in coral | Mazel et al., 2004 |
| P-462(?) | 462 | * | * | * | * | * | Platygyra daedalea | Salih et al., 2000 |
| P-505 | 505 | * | * | 492 | * | * | Plesiastrea verispora | Dove et al., 2001 |
| P-574 | 574 | 550 | * | * | * | * | Plesiastrea verispora | Dove et al., 2001 |
| P-472 | 472 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-473 | 473 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-479 | 479 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-485 | 485 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-488 | 488 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-489 | 489 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-495 | 495 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-497 | 497 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-501 | 501 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-512 | 512 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-540 | 540 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-580 | 580 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-620 | 620 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-477 | 477 | * | * | * | * | * | Plesiastrea verispora (blue) | Salih et al., 2004 |
| P-482 | 482 | * | * | * | * | * | Plesiastrea verispora (blue) | Gilmore et al., 2003 |
| P-492 | 492 | * | * | * | * | * | Plesiastrea verispora (blue) | Salih et al., 2004 |
| P-473 | 473 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-479 | 479 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-485 | 485 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-489 | 489 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-495 | 495 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-497 | 497 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-503 | 503 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-508 | 508 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-511 | 511 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-512 | 512 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-515 | 515 | * | * | * | * | * | Plesiastrea verispora (green morph) | Gilmore et al., 2003 |
| P-515 | 515 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-518 | 518 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-540 | 540 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-580 | 580 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-620 | 620 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-440 | 440 | * | * | 358 | * | * | Pocillopora damicornis | Dove et al., 2001 |
| P-499 | 499 | * | * | 484 | * | * | Pocillopora damicornis | Dove et al., 2001 |
| P-508 | 508 | * | * | 440 | 450 | * | Pocillopora damicornis | Apprill, 2003 |
| P-516 | 516 | * | * | 486 | * | * | Pocillopora damicornis | Salih et al., 2000 |
| P-625 | 625 | * | * | 570 | * | * | Pocillopora damicornis | Dove et al., 2001 |
| P-530 | 530 | * | * | 450 | ** | * | Porites astreoides | Mazel, 2003 |
| P-620 | 620 | * | * | 490 | * | * | Porites astreoides | Mazel et al., 2003 |
| P-496 | 496 | * | * | * | * | * | Porites cylindrica | Salih et al., 2000 |
| P-508 | 508 | * | * | * | * | * | Porites cylindrica | Salih et al., 2000 |
| P-485 | 485 | * | * | 420 | * | * | Porites murrayensis | Dove et al., 2001 |
| P-550 | 550 | * | * | 530 | * | * | Porites murrayensis | Dove et al., 2001 |
| P-625 | 625 | * | * | 570 | * | * | Porites murrayensis | Dove et al., 2001 |
| P-508 | 508 | * | * | 500 | * | * | Ptilosarcus sp. | Labas et al., 2002 |
| P-510 | 510 | * | * | 498 | * | * | Renilla muelleri (sea pansy) | Labas et al., 2002 |
| P-510 | 510 | * | * | * | * | * | Ricordea florida | Mazel, 1995 |
| P-513 | 513 | * | * | * | * | * | Ricordea florida | Mazel, 1995 |
| P-517 | 517 | 574 | * | 506 | 566 | * | Ricordea florida | Labas et al., 2002 |
| P-517 | 517 | * | * | 506 | * | * | Ricordea florida | Mazel, 1995 |
| P-518 | 518 | * | * | 508 | 475 | * | Ricordea florida | Labas et al., 2002 |
| P-518 | 518 | * | * | * | * | * | Ricordea florida | Mazel, 1995 |
| P-520 | 520 | * | * | * | * | * | Ricordea florida | Mazel, 1995 |
| P-573 | 573 | 510 | * | * | * | * | Ricordea florida | Mazel, 1995 |
| P-574 | 574 | 517 | * | 506 | 566 | * | Ricordea florida | Labas et al., 2002 |
| P-587-590 | 587-590 | * | * | * | * | * | Ricordea florida (mouth) | Mazel, 1995 |
| P-515 | 515 | * | * | * | * | * | Ricordea sp. | Mazel, 1995 |
| P-506 | 506 | * | * | 497 | * | * | Scolymia cubensis 1 | Labas et al., 2002 |
| P-506 | 506 | * | * | 497 | * | * | Scolymia cubensis 2 | Labas et al., 2002 |
| P-483 | 483 | 511 | * | * | * | * | Scolymia sp. | Mazel, 1995 |
| P-483 | 483 | 511 | 576 | * | * | * | Scolymia sp. | Mazel, 1995 |
| P-484 | 484 | 512 | * | * | * | * | Scolymia sp. | Mazel, 1995 |
| P-484 | 484 | 512 | 576 | * | * | * | Scolymia sp. | Mazel, 1995 |
| P-515 | 515 | * | * | * | * | * | Scolymia sp. | Mazel, 1997 |
| P-575 | 575 | 630 | * | 520 | * | * | Scolymia sp. | Mazel et al., 2003 |
| P-482 | 482 | 462 | * | * | * | * | Seriatopora hystrix | Dove et al., 2001 |
| P-503 | 503 | 535 | * | * | * | * | Siderastrea radians | Mazel, 1995 |
| P-503 | 503 | 535 | * | * | * | * | Stoichactis sp. | Mazel, 1995 |
| P-582 | 582 | * | * | 558 | * | * | Trachyphyllia geoffroyi | Ando et al., 2002 |
| P-576 | 576 | * | * | * | * | * | Zoanthus – Mature form of P-522 | Ianushevich et al., 2003 |
| P-506 | 506 | * | * | 496 | ~440 | * | Zoanthus 1 | Matz et al., 1999 |
| P-538 | 538 | ~580 | * | 528 | 494 | * | Zoanthus 2 | Matz et al., 1999 |
| P-506 | 506 | * | * | 494 | * | * | Zoanthus sp. | Yanushevich et al., 2002 |
| P-538 | 538 | * | * | 525 | 494 | * | Zoanthus sp. | Yanushevich et al., 2002 |
| *Isolated pigments – See Comments in text. | ||||||||
| **A good example of FRET – several pigments in A. millepora ‘relay’ excitation to P-593 |
||||||||
| Pigment | Emission | 2 | 3 | Excitation | 2 | 3 | Found in: | Reference |
|---|---|---|---|---|---|---|---|---|
| P-400 | 400 | * | * | 345 | * | * | Acropora horrida | Dove et al., 2001 |
| P-409 | 409 | * | * | * | * | * | Acropora nastua | Gilmore et al., 2003 |
| P-440 | 440 | * | * | 358 | * | * | Pocillopora damicornis | Dove et al., 2001 |
| P-445 | 445 | * | * | 340 | * | * | Acropora aspera | Dove et al., 2001 |
| P-446(?) | 446 | * | * | 380 | * | * | Leptoseris fragilis | Schlichter et al., 1985 |
| P-462(?) | 462 | * | * | * | * | * | Platygyra daedalea | Salih et al., 2000 |
| P-472 | 472 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-473 | 473 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-473 | 473 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-476 | 476 | 510 | 575 | 500 | 475 | * | Acropora aspera (orange band I)* | Papina et al., 2002 |
| P-477 | 477 | * | * | * | * | * | Plesiastrea verispora (blue) | Salih et al., 2004 |
| P-478 | 478 | 510 | 575 | 501 | 475 | * | Acropora aspera (orange band II)* | Papina et al., 2002 |
| P-479 | 479 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-479 | 479 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-480 | 480 | 510 | * | 481 | * | * | Acropora aspera (green band)* | Papina et al., 2002 |
| P-480 | 480 | 515 | * | 470 | 504 | * | Acropora tenuis (green band)* | Papina et al., 2002 |
| P-480 | 480 | * | * | * | * | * | Goniopora tenuidens | Salih et al., 2004 |
| P-480 | 480 | * | * | * | * | * | Montastraea annularis | Manica and Carter, 2000 |
| P-482 | 482 | * | * | * | * | * | Plesiastrea verispora (blue) | Gilmore et al., 2003 |
| P-482 | 482 | 515 | * | 451 | 430 | * | Acropora nastua | Papina et al., 2002 |
| P-482 | 482 | 462 | * | * | * | * | Seriatopora hystrix | Dove et al., 2001 |
| P-482 | 482 | 515 | * | 452 | 500 | 425 | Acropora secale (green band)* | Papina et al., 2002 |
| P-483 | 483 | * | * | 572 | 454 | 510 | Lobophyllia hemprichii (red) | Salih et al., 2004 |
| P-483 | 483 | * | * | 443 | * | * | Discosoma striata | Matz et al., 1999 |
| P-483 | 483 | 511 | * | * | * | * | Scolymia sp. | Mazel, 1995 |
| P-483 | 483 | * | * | 427 | 451 | * | Acropora nastua (green band)* | Papina et al., 2002 |
| P-483 | 483 | 511 | 576 | * | * | * | Scolymia sp. | Mazel, 1995 |
| P-484 | 484 | * | * | 456 | * | * | Clavularia sp. | Matz et al., 1999 |
| P-484 | 484 | 499 | * | * | * | * | Montastraea annularis | Mazel, 1995 |
| P-484 | 484 | 512 | * | * | * | * | Scolymia sp. | Mazel, 1995 |
| P-484 | 484 | 515 | * | 450 | 502 | * | Acropora secale | Papina et al., 2002 |
| P-484 | 484 | 512 | 576 | * | * | Scolymia sp. | Mazel, 1995 | |
| P-485 | 485 | * | * | 420 | * | * | Acropora horrida | Dove et al., 2001 |
| P-485 | 485 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-485 | 485 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-485 | 485 | * | * | 420 | 460 | 465 | Montipora caliculata | Dove et al., 2001 |
| P-485 | 485 | 517 | 555 | 465 | 505 | * | Acropora tenuis | Papina et al., 2002 |
| P-485 | 485 | * | * | 420 | * | * | Porites murrayensis | Dove et al., 2001 |
| P-486 | 486 | * | * | 384 | * | * | Acropora nobilis | Salih et al., 2000 |
| P-486 | 486 | * | * | 458 | * | * | Anemonia majano | Matz et al., 1999 |
| P-486 | 486 | * | * | 426 | * | * | Agaricia sp. | Mazel, 1997 |
| P-486 | 486 | * | * | ~448 | * | * | Diploria labyrinthiformis | Fux and Mazel, unpublished |
| P-486 | 486 | * | * | * | * | * | Montastraea annularis | Mazel, 1997 |
| P-486 | 486 | * | * | 440 | * | * | Montastraea faveolata | Lesser et al., 2000 |
| P-486 | 486 | * | * | 440 | * | * | Montastraea cavernosa | Lesser et al., 2000 |
| P-487 | 487 | 517 | * | * | * | * | Acropora cervicornis | Mazel,1995 |
| P-487 | 487 | 517 | * | * | * | * | Eusmilia fastigata | Mazel, 1995 |
| P-487 | 487 | 515 | 575 | * | * | * | Manicina areolata | Mazel, 1995 |
| P-487 | 487 | 515 | * | * | * | * | Meandrina meandrites | Mazel,1995 |
| P-488 | 488 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-489 | 489 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-489 | 489 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-490 | 490 | * | * | 425 | * | * | Acropora digitifera | Dove etal., 2001 |
| P-490 | 490 | * | * | 405 | * | * | Acropora millepora | Cox and Salih, 2005 |
| P-490 | 490 | 514 | * | 480 | 501 | * | Acropora aspera | Papina et al., 2002 |
| P-490 | 490 | * | * | 420-450 | * | * | Montipora monasteriata | Dove et al., 2001 |
| P-492 | 492 | * | * | * | * | * | Plesiastrea verispora (blue) | Salih et al., 2004 |
| P-494 | 494 | * | * | * | * | * | Goniopora tenuidens | Salih et al., 2004 |
| P-495 | 495 | * | * | 435 | * | * | Montastraea cavernosa (mc5) | Kelmanson & Matz, 2003 |
| P-495 | 495 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-495 | 495 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-495 | 495 | * | * | * | * | * | Acropora digitifera | Gilmore et al., 2003 |
| P-495 | 495 | * | * | 472 | * | * | Acropora sp. | Karasawa et al., 2003 |
| P-496 | 496 | * | * | * | * | * | Porites cylindrica | Salih et al., 2000 |
| P-496 | 496 | * | * | 399 | 482 | * | Condylactis gigantea | Labas et al., 2002 |
| P-497 | 497 | 527 | * | * | * | * | Condylactis gigantea | Mazel, 1995 |
| P-497 | 497 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-497 | 497 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-497 | 497 | 527 | * | * | * | * | Agaricia sp. | Mazel, 1995 |
| P-499 | 499 | * | * | 480 | 403 | 278 | Anemonia sculata | Wiedenmann et al., 2002 |
| P-499 | 499 | * | * | 484 | * | * | Pocillopora damicornis | Dove et al., 2001 |
| P-500 | 500 | * | * | 475 | * | * | Discosoma sp. | Gross et al., 2000 |
| P-500 | 500 | * | * | 405 | 481 | * | Heteractis crispia | Labas et al., 2002 |
| P-500 | 500 | * | * | 480 | * | * | Acropora aspera | Dove et al., 2001 |
| P-501 | 501 | * | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-503 | 503 | 479 | 578 | * | * | * | Montastraea annularis | Mazel, 1995 |
| P-503 | 503 | 535 | * | * | * | * | Siderastrea radians | Mazel, 1995 |
| P-503 | 503 | 535 | * | * | * | * | Stoichactis sp. | Mazel, 1995 |
| P-503 | 503 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-504 | 504 | * | * | 405 | * | * | Acropora millepora | Cox and Salih, 2005 |
| P-505 | 505 | * | * | 492 | * | * | Plesiastrea verispora | Dove et al., 2001 |
| P-505 | 505 | * | * | 492 | * | * | Galaxea fascicularis | Karasawa et al., 2003 |
| P-505 | 505 | * | * | * | * | * | Montastraea cavernosa | Kelmanson & Matz, 2003 |
| P-506 | 506 | * | * | 497 | * | * | Scolymia cubensis 1 | Labas et al., 2002 |
| P-506 | 506 | * | * | 497 | * | * | Scolymia cubensis 2 | Labas et al., 2002 |
| P-506 | 506 | * | * | 494 | * | * | Zoanthus sp. | Yanushevich et al., 2002 |
| P-506 | 506 | * | * | 496 | ~440 | * | Zoanthus 1 | Matz et al., 1999 |
| P-507 | 507 | 536 | 575 | * | * | * | Favia fragum | Mazel, 1995 |
| P-507 | 507 | * | * | 495 | * | * | Montastraea cavernosa (mc6) | Kelmanson and Matz, 2003 |
| P-508 | 508 | * | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-508 | 508 | * | * | 440 | 450 | * | Pocillopora damicornis | Apprill, 2003 |
| P-508 | 508 | * | * | * | * | * | Porites cylindrica | Salih et al., 2000 |
| P-508 | 508 | * | * | 494 | * | * | Dendronephthya | Labas et al., 2002 |
| P-508 | 508 | * | * | 500 | * | * | Ptilosarcus sp. | Labas et al., 2002 |
| P-508-620 | 508-620 | * | * | * | * | * | Agaricia agaricites @ 60m | Vermeij et al., 2002 |
| P-509 | 509 | * | * | * | * | * | Goniopora tenuidens | Salih et al., 2004 |
| P-509 | 509 | * | * | 397 | * | * | Aequoria victoria | Tsien, 1998 |
| P-510 | 510 | 479 | * | * | * | * | Montastraea annularis | Mazel, 1995 |
| P-510 | 510 | * | * | * | * | * | Ricordea florida | Mazel, 1995 |
| P-510 | 510 | * | * | 498 | * | * | Renilla muelleri (sea pansy) | Labas et al., 2002 |
| P-510 | 510 | * | * | 490 | 420 | * | Heteractis magnifica | Tu et al., 2003 |
| P-510 | 510 | * | * | 440 | * | * | Montastraea cavernosa | Mazel et al., 2003 |
| P-510-520 | 510-520 | * | * | 440 | * | * | Montastraea faveolata | Lesser et al., 2000 |
| P-510-520 | 510-520 | * | * | 440 | * | * | Montastraea cavernosa | Lesser et al., 2000 |
| P-510-623 | 510-623 | * | * | * | * | * | Montastraea cavernosa @ 40m | Vermeij et al., 2002 |
Transcribing the charts from various papers has been time-consuming work. The charts and the information contained within these articles are fine for our casual purposes. In all cases, the original papers should be reviewed for exact graphical data should requirements go beyond ‘aquarium’ use.

Figure 6. A beautiful Acropora specimen from the aquarium of LA hobbyist Dave Botwin. Photo by the author.
Writing this series of articles has been tedious – sometimes frustrating. It includes information I’ve gathered over the last 20 years (see the Reference list at the end of this article). I have tried to ‘weed through’ as many references as I can, looking for those one or two pertinent sentences that are often buried in pages of important, yet irrelevant, material. More often that not, what is of extreme importance to the genetic engineer is of little practical use to us as reef hobbyists. These articles are as complete as I can make them – there is always the temptation to download just one more paper, send one more email, research one ‘last’ angle. The excitement of discovery is addictive, yet at some point I must temporarily conclude the work, knowing the paper will be dated by the time I finish typing this sentence.
This time, we’ll examine pigments with emissions up to the true green portion (P-510) of the spectrum. With that said, let’s get started!
Violet and Blue Pigments
Relatively little is known about blue fluorescent coral pigments. The biomedical field has little interest in these since they do not work well in many studies due to cellular autofluorescence. In additional, the excitation wavelengths can harm living cells. Solving the issue of blue fluorescence (or blue non-fluorescence for that matter) will probably be left in the hands of coral researchers interested in these pigments’ possible ecological functions.
It is interesting to note that excitation wavelengths for P-400, P-440 and P-445 absorb some of the ultraviolet radiation in a range that natural-occurring UV sunscreens do not- MAAs or mycosporine-like amino acids absorb UV energy up to ~360nm (Bandaranayake, 1998).
P-400
Host: Acropora horrida
- Excitation: 345nm
- Emission: 400nm
- Stokes shift: 55nm
- Reference: Dove et al., 2001
- Comments: No fluorescent quantum yield is available for this pigment. Violet fluorescence is uncommon in anthozoans. This pigment’s absorption suggests exposure to UV radiation (such as that from an unshielded metal halide lamp) would result in maximum fluorescence. Salih et al., 1998 state that certain Acropora species including, among others, A. horrida and A. tortuosa, sometimes are devoid of zooxanthellae within their polyps (the symbiotic dinoflagellates are concentrated in other tissues). In these cases, the coral polyps will not contain fluorescent pigments, but coloration is found in areas where zooxanthellae are concentrated. This certainly suggests (but does not prove) that the pigments have a biological function, perhaps as a natural sunscreen. This particular Acropora specimen appeared blue in natural light.
P-409
Host: Acropora nastua
- Excitation: Not listed
- Emission: 495nm
- Stokes shift: N/A
- Reference: Gilmore et al., 2003
- Comments: This specimen appears brown with blue tips in natural light and was collected at a depth of 1.5m at Cape Maeda, Okinawa, Japan.
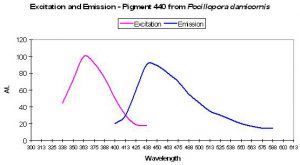
Figure 7. Excitation and emission spectra of a blue fluorescent protein found in the stony coral Pocillopora damicornis. After Dove et al., 2001.
P-440
Host: Pocillopora damicornis
- Excitation: 358nm
- Emission: 440nm
- Stokes shift: 82nm
- Reference: Dove et al., 2001
- Comments: This coral specimen appeared pink in natural light. Maximum absorption of coral tissue is 560nm, indicating the presence of a non-fluorescent chromoprotein (Dove et al., 1995). See Figure 7.
P-445
Host: Acropora aspera
- Excitation: 340nm
- Emission: 445nm
- Stokes shift: 105nm
- Reference: Dove et al., 2001
- Comments: Maximum absorption of coral tissue, zooxanthellae and pigments is at 580nm (data not shown) due to the presence of a non-fluorescent chromoprotein – pocilloporan. This Acropora specimen was blue in color. See Figure 8.
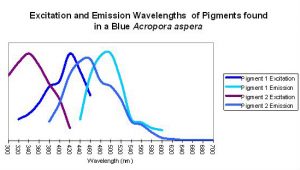
Figure 9. An apparent example of fluorescent coupling where emission spectra overlap the excitation spectra of the ‘next’ pigment. Although this coral appears blue to the eye in natural light (due to the presence of a non-fluorescent protein), its peak fluorescent emission is in the blue-green portion of the spectrum.
P-446 (?)
Although Schlichter et al. (1985, 1986) do not specifically mention the maximum fluorescence wavelength of their deep-water Leptoseris fragilis, it appears as if the fluorescent peak is close to 446nm (when variously excited by ultraviolet radiation at 380 and 390nm, violet at 400nm and blue at 430nm). The shoulder at ~380nm in Figure 10 is caused by the excitation light.
P-462 (?)
Salih et al., 2000 note a ‘dense blue’ layer of fluorescent pigment in the oral disk of a shallow-water stony coral Platygyra daedalea. No exact emission wavelength is listed, although it appears to be ~462nm in one of the article’s charts.
P-472
Host: Plesiastrea verispora
- Excitation: 330-380nm
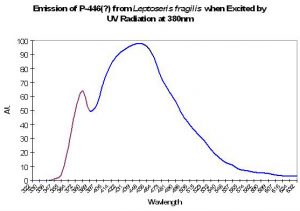
Figure 10. Emission spectrum of a deep-water coral Leptoseris fragilis. The theory of a fluorescent pigment enhancing photosynthesis was developed around this data. The peak in the emission at 380nm is actually due to the UV excitation source (this portion of the smoothed line has been color-coded violet for easy differentiation. After Schlichter et al. 1986).
- Emission: 472nm
- Stokes shift: Unknown
- Reference: Salih et al., 2004
- Comments: This P. verispora appeared blue in natural light, and was collected at a depth of 4-8m in Port Jackson, Australia.
P-473
Host: Plesiastrea verispora
- Excitation: 330-380nm
- Emission: 473nm
- Stokes shift: Unknown
- Reference: Salih et al., 2004
- Comments: These P. verispora appeared green or blue in natural light, and were collected at a depth of 4-8m in Port Jackson, Australia.
P-477
Host: Plesiastrea verispora
- Excitation: 330-380nm
- Emission: 476nm
- Stokes shift: Unknown
- Reference: Salih et al., 2004
- Comments: This P. verispora appeared blue in natural light, and was collected at a depth of 4-8m in Port Jackson, Australia.
P-479
Host: Plesiastrea verispora
- Excitation: 330-380nm
- Emission: 477nm
- Stokes shift: Unknown
- Reference: Salih et al., 2004
- Comments: These P. verispora appeared green or blue in natural light, and were collected at a depth of 4-8m in Port Jackson, Australia.
Green-Blue Pigments
P-480
Host: Acropora aspera
- Excitation: 504nm, with a shoulder at 510nm
- Emission: 480nm
- Stokes shift: 70-76nm
- Reference: Papina et al., 2002
- Comments: This emission was identified in a ‘green band’ from a SDS-PAGE separation.
P-482
Host: Acropora nastua
- Excitation: 451nm, shoulder at 430nm
- Emission: 482nm, with a shoulder at 515nm
- Stokes shift: 31nm
- Reference: Papina et al., 2002
- Comments: This Acropora nastua specimen was collected off the coast of Okinawa, Japan at a depth of 1.5m in August, 2001 (Papina et al., 2002). In daylight, the coral appeared brown with blue tips (the blue coloration probably due to a non-fluorescent chromoprotein). When viewed under a black light, the coral fluoresced blue. pH values ranging from 5.0 to 8.0 did not affect fluorescent quantum yield or spectral characteristics. See Figure 11.
Host: Seriatopora hystrix
- Excitation: 462nm
- Emission: 482nm
- Stokes shift: 20nm
- Reference: Dove et al., 2001
- Comments: Maximum absorption of coral tissue, zooxanthellae and pigments combined is at 560nm (data not shown). This coral appeared pink in natural light, due to a non-fluorescent pink pigment (one of a group of pigments collectively called pocilloporans, Dove et al., 1995). See Figure 12.
Host: Plesiastrea verispora
- Excitation: Not listed
- Emission: 482nm
- Stokes shift: N/A
- Reference: Gilmore et al., 2003.
- Comments: ThesePlesiastrea specimens (appearing blue in natural light) were collected in 5-9m of water at Port Jackson, Australia.
P-483
Host: Scolymia sp.
- Excitation: Not listed
- Emission: 483nm
- Stokes shift: N/A
- Reference: Mazel, 1995
- Comments: Emission shoulders at 511nm and 576nm. See Figure 13.
Host: Discosoma striata
- Excitation: ~458nm
- Emission: 483nm
- Stokes shift: 25nm
- Reference: Matz et al., 1999
- Comments: This pigment has a quantum yield of 0.46. Brightness (relative to the GFP in A. victoria) is 0.50. P-483 is structurally similar to P-583 (an orange-red pigment also found in some Discosoma specimens. Substitutions of only two amino acid residues in the DNA account for the shift of color from green-blue to orange-red). This pigment was concentrated in blue-green stripes on the animal’s oral disk. It apparently was in combination with an unidentified cyan fluorescent pigment.
Host: Acropora nastua
- Excitation: 427nm and 451nm
- Emission: 483nm
- Stokes shift: Unknown
- Reference: Papina et al., 2002
- Comments: This ‘green band’ was identified as an emission in a SDS-PAGE separation of pigments.
P-484
Host: Scolymia sp.
- Excitation: Not listed
- Emission: 484nm
- Stokes shift: N/A
- Reference: Mazel, 1995
- Comments: Shoulder emissions at 512nm and 576nm. See Figure 14.
Host: Acropora secale
- Excitation: 450nm, with an excitation shoulder at 502nm.
- Emission: 484nm, with a shoulder at 515nm.
- Stokes shift: 34nm
- Reference: Papina et al., 2002
- Comments: Collected in shallow water (1.5m depth) off the coast of Okinawa, Japan in August 2001. pH values ranging from 5.0 to 8.0 did not significantly affect fluorescent quantum yield or spectral absorption/emission qualities. Judging from the excitation and emission wavelengths, it appears from this pigment might be activated (probably by UV or violet/blue light) to P-515, which would change the apparent fluorescence from green-blue to more of a green coloration. The appearance of this coral was brown (due to zooxanthellae) with pink tips (probably due to the presence of the chromoprotein CP-560, a ‘pocilloporan’).
Host: Montastrea annularis
- Excitation: Not listed
- Emission: 484nm, with emission shoulder at 499nm.
- Stokes shift: N/A
- Reference: Mazel, 1995
- Comments:
Host: Clavularia sp
- Excitation: ~456nm
- Emission: 484nm
- Stokes shift: ~28nm
- Reference: Matz et al., 1999
- Comments: Fluorescent quantum yield is 0.48. Brightness (relative to the emission of A. victoria) is 0.77. As most hobbyists know, the intense green fluorescence is concentrated in the oral disk and tentacles.
P-485
Host: Acropora horrida
- Excitation: 420nm
- Emission: 485nm
- Stokes shift: 65nm
- Reference: Dove et al., 2001; Salih et al., 1999.
- Comments: Maximum absorption at 579nm (data not shown). Acropora horrida’s polyps often do not contain zooxanthellae – they, along with fluorescent coloration, are found elsewhere within the coral host’s tissues (Salih et al., 1999). This coral is blue in natural light.
Host: Montipora caliculata
- Excitation: 420nm, 450nm and 465nm
- Emission: 485nm
- Stokes shift: 20-65nm
- Reference: Dove et al., 2001
- Comments: Maximum absorption at 579nm (data not shown). This specimen was colored purple in natural light – probably as a result of the presence of a non-fluorescent chromoprotein.
Host: Porites murrayensis
- Excitation: 420nm
- Emission: 485nm
- Stokes shift: 65nm
- Reference: Dove et al., 2001
- Comments: This coral is purple in color in natural light, with a tissue maximum absorption at 576nm (data not shown). The purple coloration is likely due to the presence of a non-fluorescent chromoprotein.
Host: Plesiastrea verispora
- Excitation: 330-380nm
- Emission: 485nm
- Stokes shift: Unknown
- Reference: Salih et al., 2004
- Comments: These P. verispora appeared green or blue in natural light, and were collected at a depth of 4-8m in Port Jackson, Australia.
P-486
Host: Diploria labrynthiformis
- Excitation: Not listed
- Emission: 486nm
- Stokes shift: N/A
- Reference: Mazel, unpublished
- Comments: Specimen collected in the Caribbean.
Host: Agaricia sp.
- Excitation: 426nm
- Emission: 486nm
- Stokes shift: 60nm
- Reference: Mazel, 1997
- Comments: See Figure 16.
Host: Anemonia majano
- Excitation: 458nm
- Emission: 486nm
- Stokes shift: 28nm
- References: Matz et al., 1999; Henderson and Remington, 2005.
- Comments: Fluorescent quantum yield is 0.24. Relative Brightness (GFP from Aequorea victoria as the standard) is 0.43. Extracted pigment appears yellow-green in room lighting. Light absorbance of the wild type protein is not particularly pH-sensitive. Matz et al. mention the bright green fluorescent pigment is concentrated in the anemone’s tentacle tips. See Figure 17.
Host: Acropora nobilis
- Excitation: 384nm
- Emission: 486nm
- Stokes shift: 102nm
- Reference: Salih et al., 2000
- Comments: This Acropora specimen appeared blue when excited with UV radiation. See Figure 18.
P-487
Host: Acropora cervicornis
- Excitation: Not listed
- Emission: 487nm
- Stokes shift: N/A
- Reference: Mazel, 1997
- Comments: Has emission shoulder at 517nm. See Figure 19.
Host: Eusmilia fastigiata
- Excitation: Not listed
- Emission: 487nm
- Stokes shift: N/A
- Reference: Mazel, 1997
- Comments: See Figure 20.
Host: Meandrina meandrites
- Excitation: Not listed
- Emission: 487nm
- Stokes shift: N/A
- Reference: Mazel, 1997
- Comments:
Host: Manicina areolata
- Excitation: Not listed
- Emission: 487nm
- Stokes shift: N/A
- Reference: Mazel, 1997
- Comments: Emission shoulders were noted at 515nm and 575 nm. Though this animal contains various fluorescent compounds, it is commonly called the Rose coral for its pink/red coloration.
P-488
Host: Plesiastrea verispora
- Excitation: 330-380nm
- Emission: 488nm
- Stokes shift: Unknown
- Reference: Salih et al., 2004
- Comments: This P. verispora appeared blue in natural light, and was collected at a depth of 4-8m in Port Jackson, Australia.
P-489
Host: Plesiastrea verispora
- Excitation: 330-380nm (blue); 450-490nm (green)
- Emission: 489nm
- Stokes shift: Unknown
- Reference: Salih et al., 2004
- Comments: These P. verispora appeared blue or green in natural light, and were collected at a depth of 4-8m in Port Jackson, Australia.
P-490
Host: Acropora aspera
- Excitation: 420-450nm
- Emission: 490nm
- Stokes shift: 40-70nm
- Reference: Dove et al., 2001
- Comments: This particular coral was blue when viewed in natural light. Maximum light absorbance by tissue is ~580nm (data not shown). See Figure 21 for excitation and emission data.
Host: Acropora digitifera
- Excitation: 425nm
- Emission: 490nm
- Stokes shift: 70nm
- Reference: Dove et al., 2001
- Comments: Coral appeared purple-blue in natural light. Maximum absorbance of the tissue is at 578nm (data not shown).
Host: Acropora millepora
- Excitation: 405nm
- Emission: 490nm
- Stokes shift: 85nm
- Reference: Cox and Salih, 2005.
- Comments: Excitation via pulsed 405nm laser.
Host: Montipora monasteriata
- Excitation: 420-450nm
- Emission: 490nm
- Stokes shift: 40-70nm
- Reference: Dove et al., 2001
- Comments: Coral appeared purple in natural light. Maximum tissue absorbance equals 574-578nm (data not shown).
Blue-Green Pigments
P-492
Host: Plesiastrea verispora
- Excitation: 330-380nm
- Emission: 488nm
- Stokes shift: Unknown
- Reference: Salih et al., 2004
- Comments: This P. verispora appeared blue in natural light, and was collected at a depth of 4-8m in Port Jackson, Australia.
P-495
Host: Montastraea cavernosa
- Excitation: 435nm
- Emission: 495nm
- Stokes shift: 60nm
- Reference: Kelmanson and Matz, 2003
- Comments: M. cavernosa can contain many pigments – this one is referred to as mc5. See Figure 23.
Host: Acropora sp.
- Excitation: 472nm
- Emission: 495nm
- Stokes shift: 23nm
- Reference: Karasawa et al., 2004.
- Comments: This pigment was found in an unidentified Acropora species in the water off Okinawa, Japan. The pigment is concentrated in the polyps. It is commercially available as MiCy (Midori-ishi is Japanese for Acropora; Cy is for the color – cyan).
Host: Acropora digitifera
- Excitation: Not listed
- Emission: 495nm
- Stokes shift: N/A
- Reference: Gilmore et al., 2003
- Comments: This specimen appears brown with blue tips in natural light and was collected at a depth of 1.5m at Cape Maeda, Okinawa, Japan.
Host: Plesiastrea verispora
- Excitation: 330-380nm (blue); 450-490nm (green)
- Emission: 489nm
- Stokes shift: Unknown
- Reference: Salih et al., 2004
- Comments: These P. verispora appeared blue or green in natural light, and were collected at a depth of 4-8m in Port Jackson, Australia.
P-496
Host: Condylactis gigantea
- Excitation: 399nm, with a shoulder at 482nm.
- Emission: 496nm
- Reference: Labas et al., 2002
- Comments: P-496 is found in the anemone Condylactis gigantea (Labas 2002). These anemones are popular in aquaria, and for good reason. They are easy to maintain in captivity and coloration can be spectacular. It is not uncommon to see them with green/purple tentacles and contrasting magenta or purple tips (Sprung and Delbeek, 1997). The column or stalk can be orange or yellow and is due to non-fluorescent chromoproteins. However, fluorescence is blue-green. Labas et al. (2002) believe the double peaked excitation spectrum (similar to wild-type GFP) suggests that the pigment is photoconvertible, though this opinion is the subject of debate (Mazel, personal communication; Tsien, 1998). See Figure 24.
P-497
Host: Condylactis gigantea
- Excitation: Not listed
- Emission: 497nm
- Stokes shift: N/A
- Reference: Mazel, 1995
- Comments: The Atlantic anemone’s fluorescent emission also has a shoulder emission at 527nm. See Figure 25.
Host: Agaricia sp.
- Excitation: Not listed
- Emission: 497nm
- Stokes shift: N/A
- Reference: Mazel, 1995
- Comments:
Host: Plesiastrea verispora
- Excitation: 330-380nm (blue); 450-490nm (green)
- Emission: 489nm
- Stokes shift: Unknown
- Reference: Salih et al., 2004
- Comments: These P. verispora appeared blue or green in natural light, and were collected at a depth of 4-8m in Port Jackson, Australia.
P-499: Precursor to P-522
Host: Anemonia sculata
- Excitation: 278, 403 and 480nm
- Emission: 499nm, with shoulder at 522nm.
- Stokes shift: 19nm
- Reference: Wiedenmann et al., 2000; Labas et al., 2002; Verkhusha et al., 2001
- Comments: The green fluorescent protein is apparently the immature form of P-522.
Host: Pocillopora damicornis
- Excitation: 484nm
- Emission: 499nm
- Stokes shift: 55nm
- Reference: Dove et al., 2001
- Comments: This P. damicornis appeared pink in natural light. Maximum tissue absorption equals 560nm (data not shown), but see Figure 26 for fluorescence information.
P-500: Possible Precursor of Pigment 580
Host: Acropora aspera
- Excitation: 480nm
- Emission: 500nm
- Stokes shift: 20nm
- Reference: Dove et al., 2001
- Comments: Maximum tissue absorbance is at 580nm (data not shown). This coral appears blue in natural light.
Host: Heteractis crispa
- Excitation: 405nm, with a shoulder at 481nm.
- Emission: 500nm
- Stokes shift: 95nm
- Reference: Labas et al., 2002
- Comments: Double peaked excitation suggests that photoconversion is possible (Labas et al., 2002) – again, this is a matter of debate in the scientific community. Heteractis also has a non-fluorescent chromoprotein.
Host: Discosoma sp.
- Excitation: 475nm
- Emission: 500nm
- Stokes shift: 25nm
- Reference: Gross et al., 2000.
- Comments: Minor substitutions in the residue via mutagenesis prevent maturation.
P-501
Host: Plesiastrea verispora
- Excitation: 450-490nm
- Emission: 501nm
- Stokes shift: Unknown
- Reference: Salih et al., 2004
- Comments: This Plesiastrea appeared blue (likely due to the presence of a pocilloporan protein) and was collected in 4-8m of water at Port Jackson, Australia.
P-503
Host: Montastrea annularis
- Excitation: Not listed
- Emission: 503nm, with shoulders at 479nm and 578nm
- Stokes shift: N/A
- Reference: Mazel, 1995
- Comments:
Host: Siderastrea radians
- Excitation: Not listed
- Emission: 503nm
- Stokes shift: N/A
- Reference: Mazel, 1995
- Comments:
Host: Stoichactis sp.
- Excitation: Not listed
- Emission: 503nm, shoulder at 535nm
- Stokes shift: N/A
- Reference: Mazel, 1995
- Comments: Giant Carpet anemones (Stoichactis) are green, purple, pink, blue or fluorescent reddish pink- Sprung and Delbeek, 1997.
Host: Plesiastrea verispora
- Excitation: 450-490nm
- Emission: 503nm
- Stokes shift: Unknown
- Reference: Salih et al., 2004
- Comments: This Plesiastrea appeared green in natural light and was collected in 4-8m of water at Port Jackson, Australia.
P-504
Host: Acropora millepora
- Excitation: 405nm
- Emission: 504nm
- Stokes shift: 99nm
- Reference: Cox and Salih, 2005.
- Comments: Excitation via pulsed 405nm laser.
P-505
Host: Montastraea cavernosa
- Excitation: Not listed
- Emission: 505nm
- Stokes shift: N/A
- Reference: Kelmanson and Matz, 2003.
Host: Plesiastrea verispora
- Excitation: 492nm
- Emission: 505nm
- Stokes shift: 13nm
- Reference: Dove et al., 2001
- Comments: This Plesiastrea specimen appeared green in natural light. See Figure 28.
Host: Galaxea fascicularis
- Excitation: 492nm
- Emission: 505nm
- Stokes shift: 13nm
- Reference: Karasawa et al., 2003.
- Comments: This pigment is commercially available as “Azami-Green” (the Japanese name for Galaxea is “Azami-Sango”). Galaxea specimens, despite their aggressive nature through use of long sweeper tentacles, are often kept in reef aquaria. See Figure 29.
P-506
Host: Zoanthus 1
- Excitation: 492nm
- Emission: 506, with shoulder at ~540nm
- Stokes shift: 14nm
- Reference: Matz et al., 1999.
- Comments: Fluorescent quantum yield = 0.63; Relative Brightness =1.02 (meaning it is just slightly brighter than the A. victoria GFP reference standard). See Figure 30.
Host: Scolymia cubensis
- Excitation: 497nm
- Emission: 506nm
- Stokes shift: 9nm
- Reference: Labas et al., 2002.
- Comments: Listed as GFPs 1 and 2 from Scolymia cubensis by Labas et al., 2002.
P-507
Host: Favia fragum
- Excitation: Not listed
- Emission: 507nm, shoulders at 536nm and 575nm
- Stokes shift: N/A
- Reference: Mazel, 1995
- Comments:
Host: Montastraea cavernosa
- Excitation: Not listed
- Emission: 507nm
- Stokes shift: N/A
- Reference: Kelmanson and Matz, 2003.
- Comments: M. cavernosa can contain many pigments – this one is referred to as mc6. See Figure 31.
P-508: Precursor of Pigment 575
Host: Dendronephthya sp.
- Excitation: 494nm
- Emission: 508nm
- Stokes shift: 14nm
- Reference: Labas et al., 2002
- Comments: P-508 matures from green to red under intense blue light (Labas 2002) even in anaerobic conditions, but not in the dark – light mediates the change in the process known as photoconversion (Nienhaus et al., 2005). It is interesting that a fluorescent pigment found in a coral void of zooxanthellae (especially one thought capable of photoconversion when exposed to blue light) is considered by some to act as a photoprotectant. This pigment is commercially available as Dendra (‘Dend’ for Dendronephthya and ‘ra’ for red activatable). See Figure 32.
Host: Porites cylindrica
- Excitation: Not listed
- Emission: 508nm
- Stokes shift: N/A
- Reference: Salih et al., 2000
- Comments: Emission shoulders at 496 and 508nm (Salih et al., 2000).
Host: Ptilosarcus guernyi (Sea Pen)
- Excitation: 500nm
- Emission: 508nm
- Stokes shift: 8nm
- Reference: Labas et al., 2002; Peele et al., 2001
- Comments: These Sea Pens are often found on soft or muddy bottoms at depths to ~75m. This is yet another example of a fluorescent pigment within an azooxanthellate cnidarian. This GFP is believed by some to be of very early origin, and perhaps a precursor to many of the fluorescent pigments seen today.
P-509: The ‘Original’ Green Fluorescent
Protein
The ‘original’ green fluorescent protein (the widely accepted name and abbreviated as GFP) was isolated from tissues of the jellyfish Aequorea victoria and, later, the Sea Pen Renilla reniformis. Although it was described early on, P-509 has not been placed with sufficient confidence within the coral pigment phylogenetic tree and is therefore not positively described as an ancestreal form (Labas et al., 2002). Years of attempts to engineer P-509 to fluoresce far-red shifted wavelengths have failed. However, P-509’s absorption characteristics are altered by exposure to wavelengths 400nm and shorter (meaning ultraviolet radiation). With this pigment, we have more information on the potential effects of light energy at relatively narrow bandwidth on the spectral characteristics. Absorbance increases at 478nm (and decreases at 398nm) when irradiated with monochromatic light of 398nm. Interestingly, absorbance increases at 478nm when the pigment is excited by the same wavelength. This process can take hours, and recovery is at about 60% after 24 hours (Chattoraj et al., 1996; See Figure 34.).
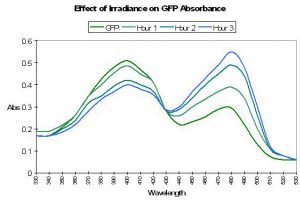
Figure 34. Ultraviolet irradiation (at 398nm) causes a shift in absorbance of Aequorea victoria’s GFP. See text for details. After Chattoraj et al., 1996.
Host: Aequorea victoria (Jellyfish)
- Excitation: 397nm
- Emission: 509nm
- Stokes shift: 112nm
- Reference: Tsien, 1998.
- Comments: Discovered by Osamu Shimomura et al. in the jellyfish Aequorea victoria during the early 1960’s (Shimomura et al., 1962). Formation of the fluorophore requires molecular oxygen and apparently does not require enzymes or cofactors (Heim et al., 1994). Emission maximum is sometimes listed as 508nm. Emission is 503nm when excited by green-blue light at 476nm. See Figure 33.
Host: Renilla reniformis (Sea Pansy)
- Excitation: 498nm
- Emission: 509nm
- Stokes shift: 11nm
- Reference: Labas et al., 2002
- Comments: Emission variously listed as 509nm and 510nm.
P-510
Host: Montastraea annularis
- Excitation: 440nm
- Emission: 510nm
- Stokes shift: 70nm
- Reference: Mazel et al., 2003.
- Comments: Shoulder at 479 nm. See Figure 35.
Host: Ricordea florida
- Excitation: Not listed
- Emission: 510nm
- Stokes shift: N/A
- Reference: Mazel, 1995
- Comments: Shoulder at 479 nm.
The quick glance at Pigment 510 concludes Part 1 of this series. Next time, we’ll examine some of the truly green and other variously colored fluorescent pigments. We’ll also see some confirmed examples of photoconversion. If you’re curious about coloration, this promises to get pretty interesting.
For convenience (mostly mine), I have chosen to list below all references cited in this series of articles. If you’re interested in discussing coral coloration, please email me at [email protected].
Acknowledgement
Many mahalos to Charles Mazel for his time and helpful comments during the preparation of this article. See his website at www.nightsea.com.
References and Further Reading
- Ando, R., H. Hama, M. Yamamoto-Hino, H. Mizuno, and A. Miyawaki, 2002. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. USA, 99(20):12651-12656.
- Ando, R., H. Mizuno and A. Miyawaki, 2004. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science, 306:1370-1373.
- Andresen, M., M. Wahl, A. Stiel, F. Gräter, L. Schäfer, S. Trowitzsch, G. Weber, C. Eggeling, H. Grubmüller, S. Hell and S. Jakobs, 205. Structure and mechanism of the reversible photoswitch of a fluorescent protein. Proc. Natl. Acad. Sci. USA, 102, 37:13070-13074.
- Apprill, A., 2003. Spectral characteristics and genetic expression of green fluorescent proteins in Hawaiian corals. In: Molecular Biology of Corals: Results of 2002 Edwin W. Pauley Summer Program in Marine Biology, E. Cox and T. Lewis, eds. University of Hawaii HIMB Technical Report No. 43:6-13.
- Baird, G., D. Zacharias and R. Tsien, 2000. Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proc. Natl. Acad. Sci. USA, 97(22):11984-11989.
- Bandaranayake, W., 1998. Mycosporines: Are they nature’s sunscreens? National Product Review, 1998. 159-172.
- Bingman, C., 1995. Green-fluorescent protein: a model for coral host fluorescent proteins? Aquarium Frontiers, 2(3): 6-9.
- Bingman, C., 1999. Biochemistry of Aquaria: Coral Fluorescence – An Update. http://www.reefs.org/library/aquarium_frontiers/index.html
- Blundell, A., 2005. Lateral Lines: The Seen and Unseen World of Coral Fluorescence. http://www.advancedaquarist.com/2005/2/lines/
- Bulina, M., D. Chudakov, N. Mudrik, and K. Lukyanov, 2002. Interconversion of Anthozoa GFP-like fluorescent and non-fluorescent proteins by mutagenesis. BMC Biochem., 24; 3(1):7.
- Bulina, M., K. Lukyanov, I. Yampolsky, D. Chudakov, D. Staroverov, A. Shcheglov, N. Gurskaya and S. Lukyanov, 2004. New class of blue animal pigments based on frizzled and kringle protein domains. J. Biol. Chem., 279(42):43367-43370.
- Burr, A., P. Hunt, D. Wagar, S. Dewilde, M. Blaxter, J. Vanfleteren and L. Moens, 2000. A hemoglobin with an optical function. J. Biol. Chem., 275(7): 4810-4815.
- Calfo, A., 2005. Magnificent fluorescence! Aquaristic perspectives. http://reefkeeping.com/issues/2005-11/ac/index.php
- Chattoraj, M., B. King, G. Bublitz and S. Boxer, 1996. Ultra-fast excited state dynamics in green fluorescent protein: Multiple states and proton transfer. Proc. Natl. Acad. Sci. USA, 93: 8362-8367.
- Chudakov, D., A. Feofanov, N. Mudrik, S. Lukyanov and K. Lukyanov, 2003. Chromophore environment provides clue to “kindling fluorescent protein” riddle. J. Biol. Chem., 278(9):7215-7219.
- Chudakov, D., V. Belousov, A. Zararisky, V. Novoselov, D. Staroverov, D. Zorov, S. Lukyanov and K. Lukyanov, 2003. Kindling fluorescent proteins for precise in vivo photolabeling. Nat. Biotechnol. 21(2): 191-194.
- Cotlet, M., J. Hofkens, S. Habuchi, G. Dirix, M. Van Guyse, J. Michiels, J. Vanderleyden and F. De Schryver, 2001. Identification of different emitting species in the red fluorescent protein DsRed by means of ensemble and single-molecule spectroscopy. Proc. Natl. Acad. Sci. USA, 98(25):14398-14403.
- Cox, G. and A. Salih, 2005. Fluorescent lifetime imaging of symbionts and fluorescent proteins in reef corals. In: Multiphoton Microscopy in the Biomedical Sciences V, edited by A. Periasami and Peter So. Proc. SPIE, 5700:162-170.
- Credabel, J., 2006. Notes from the trenches: Coral fusion and grafting. http://reefkeeping.com/issues/2006-02/nftt/index.php
- D’Elia, C., S. Domotor and K. Webb, 1983. Nutrient uptake kinetics of freshly isolated zooxanthellae. Mar. Biol., 75:157-167.
- Delbeek, J. and J. Sprung, 1994. The Reef Aquarium: A Comprehensive Guide to the Identification and Care of Tropical Marine Invertebrates. Vol. 1. Ricordea Publishing, Coconut Grove, Fl. 544 pp.
- Delbeek, J. and J. Sprung, 2005. The Reef Aquarium: Science, Art and Technology. Vol. 3. Ricordea Publishing, Coconut Grove, Fl. 679 pp.
- Delbeek, J.C., 2003. Media Review, Advanced Aquarist Online Magazine, June. www.advancedaquarist.com/issues/june2003/media.htm
- Dittrich, P., S. Schäfer and P. Schwille, 2005. Characterization of the photoconversion on reaction of the fluorescent protein Kaede on the single-molecule level. Biophys. J., 89:3446-3455.
- Dove, S., M. Takabayashi and O. Hoegh-Guldberg, 1995. Isolation and partial characterization of the pink and blue pigments of Pocilloporid and Acroporid corals. Biol. Bull., 189:288-297.
- Dove, S., O. Hoegh-Guldberg and S. Ranganathan, 2001. Major color patterns of reef-building corals are due to a family of GFP-like proteins. Coral Reefs 19: 197-204.
- Dove, S., 2004. Scleractinian corals with photoprotective host pigments are hypersensitive to thermal bleaching. Mar. Ecol. Prog. Ser., 272: 99-116.
- Dove, S., J. Oritz, S. Enriquez, M. Fine, P. Fisher, R. Iglesias-Prieto, D. Thornhill and O. Hoegh-Guldberg, 2006. Response of holosymbiont pigments from the scleractinian coral Montipora monasteriata to short-term heat stress. Limnol. Oceanogr., 51(2): 1149-1158.
- Elowitz, M., M. Surette, P. Wolf, J. Stock and S. Leibler, 1997. Photoactivation turns green fluorescent protein red. Curr. Biol., 7(10):809-812.
- Fabricius, F., 2006. Effects of irradiance, flow and colony pigmentation on the temperature microenvironment around corals: Implications for coral bleaching. Limnol. Oceanogr., 51(1): 30-37.
- Finet, B. and F. Lesage, 2005. Colors by the thousands – Light, Colors and Corals, Part 1.
- Advanced Aquarist Online, December 2005. http://www.advancedaquarist.com/2005/12/aafeature2
- Fitt, W.K., T.A.V. Rees and D. Yellowlees, 1995. Relationship between pH and the availability of dissolved inorganic nitrogen in the zooxanthella-giant clam symbiosis. Limnol. Oceanogr., 40(5): 976-982.
- Fox, D.L. and D.W. Wilkie, 1970. Somatic and skeletally fixed carotenoids of the purple hydrocoral, Allopora californica. Comp. Biochem. Physiol., 36:49-60.
- Fox, D.L., 1972. Pigmented calcareous skeletons of some corals. Comp. Biochem. Physiol., 43B:919-927.
- Fux, E. and C. Mazel, Unpublished. An experimental method to separate the fluorescence and reflectance components of the spectral signatures of corals.
- Fux, E. and C. Mazel, 1999. Unmixing coral fluorescence emission spectra and predicting new spectra under different excitation conditions. Applied Optics. 38, 3: 486-494.
- Garcia-Parajo, M., M. Koopman, E. van Dijk, V. Subramaniam, and N.F. van Hulst, 2001. The nature of fluorescence emission in the red fluorescent protein DsRed, revealed by single-molecule detection. Proc. Natl. Acad. Sci. USA, 98(25):14392-14397.
- Gentien, P., 1981. Fluorescent metabolites in coral reefs off Townsville, Queensland. Aust. J. Mar. Freshwater Res., 32: 975-980.
- Gilmore, A., A. Larkum, A. Salih, S. Itoh, Y. Shibata, C. Bena, H. Yamasaki, M. Papina and R. van Woesik, 2003. Simultaneous time resolution of the emission spectra of fluorescent proteins and zooxanthellar chlorophyll in reef-building corals. Photochem. Photobiol., 77(5): 515-523.
- Gorbunov, M. and P. Falkowski, 2002. Photoreceptors in the cnidarian hosts allow symbiotic corals to sense blue moonlight. Limnol. Oceanogr., 47(1): 309-315.
- Gross, L., G. Baird, R. Hoffman, K. Baldridge and R. Tsien, 2000. The structure of the chromophore with DsRed, a red fluorescent protein from coral. Proc. Natl. Acad. Sci. USA, 97(22): 11990-11995.
- Gulko, D., M. Lesser and M. Ondrusek, 1995. Introduction of materials and methods commonly used by participants in the 1994 HIMB summer program on UV radiation and coral reefs. . In: Ultraviolet Radiation and Coral Reefs. D. Gulko and P.L. Jokiel (eds.), HIMB Technical Report #41, 19-23.
- Gurskaya, N., A. Fradkov, A. Terskikh, M. Matz, Y. Labas, V. Martynov, Y. Yanushevich, K. Lukyanov and S. Lukyanov, 2001. GFP-like chromoproteins as a source of far-red fluorescent proteins. FEBS Letters, 507(1):16-20.
- Gurskaya, N., V. Verkhusha, A. Shcheglov, D. Staroverov, T. Chepurnykh, A. Fradkov, S. Lukyanov and K. Lukyanov, 2006. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nature Biotechnology, 24: 461-465.
- Heim, R., D. Prashner and R. Tsien, 1994. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc. Natl. Acad. Sci. USA, 91: 12501-12504.
- Henderson, J. and S. Remington, 2005. Crystal structures and mutational analysis of amFP486, a cyan fluorescent protein from Anemonia majano. Proc. Natl. Acad. Sci. USA, 102, 36: 12712-12717.
- Hochberg, E., M. Atkinson, A. Apprill and S. Andrèfouët, 2004. Spectral reflectance of coral. Coral Reefs, 23: 84-95.
- Hollingsworth, L., R. Kinzie III, T. Lewis, D. Krupp and J-AC Leong, 2005. Photoaxis of motile zooxanthellae to green light may facilitate symbiont capture by coral larvae. Coral Reefs, 24: 523.
- Ianushevich, I., N. Gurskaia, D. Staroverov, S. Lukyanov and K. Lukyanov, 2003. A natural fluorescent protein that changes its fluorescence during maturation. Bioorg Khim., 29(4):356-360.
- Ip, D., S-H Chan, M. Allen, M. Bycroft, D. Wan and K-B Wong, 2004. Crystallization and preliminary crystallographic analysis of a novel orange fluorescent protein from the cnidaria tube anemone Cerianthus. Acta Crystallogr., Section D, 60:340-341.
- Israel, A. and S. Beer, 1992. Photosynthetic carbon acquisition in the red alga Gracilaria conferta. 2. Rubisco carboxylase kinetics, carbonic anhydrase, and bicarbonate uptake. Mar. Biol., 112:697-700.
- Karasawa, S., T. Araki, M. Yamamoto-Hino, and A. Miyawaki, 2003. A green-emitting fluorescent protein from Galaxeidae coral and its monomeric use in fluorescent labeling. J. Biol. Chem., 278:34167-34171.
- Karasawa, S., T. Araki, T. Nagai, H. Mizuno and A. Miyawaki, 2004. Cyan-emitting and orange-emitting fluorescent proteins as a donor/acceptor pair for fluorescent resonance energy transfer. Biochem. J., 381(Part 1):307-312.
- Khang, S. and A. Salih, 2005. Localization of fluorescent pigments in a nonbioluminescent, azooxanthellate octocorals suggests a photoprotective function. Coral Reefs, 24, 3: 435.
- Kawaguti, S., 1937. On the physiology of reef corals II. The effect of light on colour and form of reef corals. Palao Trop. Biol. Sta. Taihoku Imperial University, Taihoku. 2.
- Kawaguti, S., 1944. On the physiology of reef corals VI. Study on the pigments. Palao Trop. Biol. Sta. Study, 2:617-674.
- Kawaguti, S., 1966. Electron microscopy on the fluorescent green of reef corals with a note on the mucous cells. Biol. J. Okayama Univ., 12:11-21.
- Kelmanson, I. and M. Matz, 2003. Molecular basis and evolutionary origins of color diversity in Great Star coral Montastraea cavernosa (Scleractinia:Faviida). Mol. Biol. Bull., 20, 7: 1125-1133.
- Kennedy, G.Y., 1979. Pigments of marine invertebrates. Adv. Mar. Biol., 16:309-381.
- Kinzie, R.A. and T. Hunter, 1987. Effect of light quality on photosynthesis of the reef coral Montipora verrucosa. Mar. Biol., 94:95-109.
- Kinzie, R.A., P.L. Jokiel and R. York, 1984. Effects of light of altered spectral composition on coral zooxanthellae associations and on zooxanthellae in vitro. Mar. Biol., 78:239-248.
- Kirk, J.T.O., 1983. Light and Photosynthesis in Aquatic Ecosystems. Cambridge University Press, Cambridge. 401 pp.
- Koh, E., 1997. Secretion of bioactive compounds by a scleractinian coral. Proc. 8th Int. Coral Reef Symp., 2:1263-1266.
- Kutser, T., A. Dekker and W. Skirving, 2003. Modeling spectral discrimination of Great Barrier Reef benthic communities by remote sensing instruments. Limnol. Oceanogr., 48(1, Part 2): 497-510.
- Labas, Y., N. Gurskaya, Y. Yanushevich, A. Fradkov, K. Lukyanov, S. Lukyanov, and M. Matz, 2002. Diversity and evolution of the green fluorescent protein family. Proc. Natl. Acad. Sci. USA. 99 (7): 4256-4261.
- Larkum, A., G. Cox, R. Hiller, D. Parry and T. Dibbayawan, 1987. Filamentous cyanophytes containing phycocourobilin and in symbiosis with sponges and an ascidian of coral reefs. Mar. Biol., 95(1):1-13.
- Lesser, M., C. Mazel, D. Phinney and C. Yentsch, 2000. Light absorption and utilization by colonies of the congeneric hermatypic corals Montastraea faveolata and Montastraea cavernosa. Limnol. Oceanogr., 45(1): 76-86.
- Logan, A., K. Halcrow and T. Tomascik, 1990. UV excitation-fluorescence in polyp tissue of certain scleractinian corals from Barbados and Bermuda. Bull. Mar. Sci., 46(3):807-813.
- Louchard, E., R. Reid, C. Stephens, C. Davis, R. Leathers and T. Downes, 2003. Optical remote sensing of benthic habitats and bathymetry in coastal environments at Lee Stocking Island, Bahamas: A comparative spectral classification approach. Limnol. Oceanogr., 48(1):511-521.
- Lukyanov, K., A. Fradkov, N. Gurskaya, M. Matz, Y. Labas, A. Savitsky, M. Markelov, A. Zaraisky, X. Zhao, Y. Fang, W. Tan and S. Lukyanov, 2000. Natural animal coloration can be determined by a non-fluorescent green fluorescent protein homolog. J. Biol. Chem., 275(34):25879-25882.
- Manica, A. and R. Carter, 2000. Morphological and fluorescent analysis of the Montastraea annularis species complex in Florida. Mar. Biol., 137(5-6):899-906.
- Martynov, V., B. Maksimov, N. Martynova, A. Pakhomov, N. Gurskaya and S. Lukyanov, 2003. A purple-blue chromoprotein from Goniopora tenuidens belongs to the DsRed subfamily of GFP-like proteins. J. Biol. Chem., 278(47):46288-46292.
- Martynov, V., A. Savitsky, N. Martynov, P. Savitsky, K. Lukyanov and S. Lukyanov, 2001. Alternative cyclization in GFP-like proteins family. The formation and structure of the chromophore of a purple chromoprotein from Anemonia sculata. J. Biol. Chem., 276(24):21012-21016.
- Matz, M., A. Fradkov, Y. Labas, A. Savitsky, A. Zaraisky, M. Markelov and S. Lukyanov, 1999. Fluorescent proteins from nonbioluminescent Anthozoa species. Nature Biotechnology, 17:969-973.
- Matz, M., 2002. Diversity and evolution of the green fluorescent protein family. Proc. Natl. Acad. Sci. USA. 99 (7): 4256-4261.
- Matz, M., K. Lukyanov and S. Lukyanov, 2002. Family of the green fluorescent protein: Journey to the end of the rainbow. Bioessays 24(10):953-959.
- Matz, M., N. Marshall and M. Vorobyev, 2006. Are corals colorful? J. Am. Soc. Photochem. Photobiol., 82(2):345-350.
- Mazel, C.H., 1995. Spectral measurements of fluorescence emission in Caribbean cnidarians. Mar. Ecol. Prog. Ser., 120:185-191.
- Mazel, C.H., 1997. Coral fluorescence characteristics: excitation – emission spectra, fluorescence efficiencies,and contribution to apparent reflectance. Ocean Optics XIII. 240-245.
- Mazel, C., M. Lesser, M. Gorbunov, T. Barry, J. Farrell, K. Wyman and P. Falkowski, 2003. Green fluorescent proteins in Caribbean corals. Limnol. Oceanogr., 48(1, part 2), 402-411.
- Mazel, C., M. Lesser, M. Gorbunov and P. Falkowski, 2004. Discovery of nitrogen-fixing cyanobacteria in corals. Science, 305: 997-1000.
- Mazel, C. H., and E. Fuchs, 2003. Contribution of fluorescence to the spectral signature and perceived color of corals. Limnol. Oceanogr. 48:390-401.
- Mazel, C.H., 2005. Underwater fluorescence photography in the presence of ambient light. Limnol. Oceanogr.: Methods
3:499-510. - Minghelli-Roman, A., J. Chisolm, M. Marchioretti, and J. Jaubert, 2002. Discrimination of coral reflectance spectra in the Red Sea. Coral Reefs, 21(3):307-314.
- Miyawaki, A., 2002. Green fluorescent protein-like proteins in reef Anthozoa animals. Cell Struct. Func., 27:343-347.
- Moore, L. and S. Chisolm, 1999. Photophysiology of the marine cyanobacterium Prochlorococcus: Ecotypic differences among cultured isolates. Limnol. Oceanogr., 44(3):628-638.
- Moseley, H., 1877. On the colouring matter of various animals and especially of deep-sea forms dredged by the H.M.S. Challenger. Quarterly Journal of the Microscopical Society, 17: 1-23.
- Myers, M., J. Hardy, C. Mazel, and P. Dustan, 1999. Optical spectra and pigmentation of Caribbean reef corals and macroalgae. Coral Reefs 18: 2, 179-186.
- Nienhaus, K., B. Vallone, F. Renzi, J. Wiedenmann and G. Nienhaus, 2003. Crystallization and preliminary X-ray diffraction analysis of the red fluorescent protein eqFP611. Acta Cryst., D59:1253-1255.
- Nienhaus, K., G. Nienhaus, J. Wiedenmann and H. Nar, 2005. Structural basis for photo-induced protein cleavage and green-to-red conversion of fluorescent protein EosFP. Proc. Natl. Acad. Sci., USA, 102(26):9156-9159.
- Neveux, J., M. Tenório, C. Dupouy and T. Villareal, 2006. Spectral diversity of phycoerythrins and diazotroph abundance in tropical waters. Limnol. Oceanogr., 51(4):1689-1698.
- Pakahomov, A., N. Martynova, N. Gurskaya, T. Balashova and V. Martynov, 2004. Photoconversion of the chromophore of a fluorescent protein from Dendronephthya sp. Biochem. (Mosc.), 69(8):901-908.
- Papina, M., Y. Sakihama, C. Bena, R. van Woesik and H. Yamasaki, 2002. Separation of highly fluorescent proteins by SDS-PAGE in Acroporidae corals. In press, Comp. Biochem. Physiol.
- Paringit, E. and K. Nadaoka, 2001. Development of a canopy reflectance model for coral reef areas: Inferences from field spectral measurements and modeling efforts. Proc. 22nd Asian Conference on Remote Sensing.
- Pasternak, Z., B. Blasius, A. Abelson and Y. Achituv, 2006. Host-finding behavior and navigation capabilities of symbiotic zooxanthellae. Coral Reefs, 25(2):201-207.
- Pederson, H., 1968. Fluorescent flowers of the sea. Sea Frontiers, 14(4): 194-199.
- Peelle, B., T. Gururaja, D. Payan and D. Anderson, 2001. Characterization and use of green fluorescent proteins from Renilla mulleri and Ptilosarcus guernyi for the human cell display of functional peptides. J. Protein Chem., 20(6):507-19.
- Pieribone, V. and D. Gruber, 2005. Aglow in the Dark: The Revolutionary Science of Biofluorescence. The Belknap Press of Harvard University Press, Cambridge Massachusetts, and London, England. 263 pp.
- Piniak, G., N. Fogarty, C. Addison and W. Kenworthy, 2005. Fluorescence census techniques for coral recruits. Coral Reefs, 24: 496-500.
- Prashner, D., V. Enckenrode, W. Ward, F. Pentegast and M. Cormier, 1992. Primary structure of the Aequorea victoria green-fluorescent protein. Gene, 111: 229-233.
- Riddle, D. and A. Amussen, 1998. Coral pigments. Online presentation. http://www.reefs.org/library/talklog/d_riddle_042698.html
- Riddle, D., 2003. Effects of narrow bandwidth light sources on coral host and zooxanthellae pigments. Advanced Aquarist Online, November 2003. http://www.advancedaquarist.com/issues/nov2003/feature.htm
- Riddle, D., 2006. Product Review: Ocean Optics spectrometers and software. Advanced Aquarist Online, June 2006. http://www.advancedaquarist.com/2006/6/review
- Rinkevich, B. and Y. Loya, 1983. Intraspecific competition networks in the Red Sea coral Stylophora pistillata. Coral Reefs, 1:161-172.
- Salih, A., 2003. An exploration of light regulating pigments of reef-building corals from macro- to micro- and nano-scales. In: From Zero to Infinity, J. Nicholls and B. Pailthorpe, eds. The Science Foundation for Physics, University of Sydney. Chapter 4:49-69.
- Salih, A., A. Larkum, T. Cronin, J. Wiedenmann, R. Szymczak and G. Cox, 2004. Biological properties of coral GFP-type proteins provide clues for engineering novel optical probes and biosensors. In: Genetically Engineered and Optical Probes for Biomedical Applications II, A. Savitsky et al., eds. Proc. of SPIE, 5329:61-72.
- Salih, A., O. Hoegh-Guldberg and G. Cox, 1998. Photoprotection of symbiotic dinoflagellates by fluorescent pigments in reef corals. Proc. Australian Coral Reef Society, 75th Anniversary Conference, Heron Island, Australia. 217-230.
- Salih, A., A. Larkum, G.Cox, M.Kuhl and O.Hoegh-Guldberg, 2000. Fluorescent pigments in corals are photoprotective. Nature, 408: 850-853.
- Salih A, Larkum AWD & G Cox, 2001. Photoprotection from photoinhibition of symbiotic algae in corals by fluorescent pigments. 12th International Congress on Photosynthesis, Brisbane, Australia.
- Salih, A., G. Cox and A. Larkum, 2003. Cellular organization and spectral diversity of GFP-like proteins in live coral cells studied by single and multiphoton imaging and microspectroscopy. In: Multiphoton Microscopy in the Biomedical Sciences III, A. Periasamy and P. So, Editors. Proc. SPIE, Vol. 4963: 194-200.
- Salih, A, Larkum AWD & G Cox, 2001. Corals use fluorescent pigments as natural sunscreens. Australian Coral Reef Society Conference, Magnetic Island, Australia.
- Salih, A, Cox G & AWD Larkum, 2000. The role of fluorescent pigments: evidence of enhanced resistance to bleaching in fluorescently pigmented corals. IX International Coral Reef Symposium, Bali, Indonesia.
- Salih, A., G. Cox & AWD Larkum, 2000. Energy dissipation by fluorescence coupling by coral fluorescent pigments. Optical Society Conference, Application of Optical Techniques in Biological Sciences.
- Salih, A., Hoegh-Guldberg O, Cox G & AWD Larkum, 1999. Protection against bleaching of corals by fluorescent pigments during the 1998 mass bleaching event. XIX Pacific Science Congress.
- Salih A., Hoegh-Guldberg, O. & Cox G., 1999. Are fluorescent colors in corals photoprotective? Australian Coral Reef Society Conference.
- Salih, A., Hoegh-Guldberg, O. & Cox G., 1998. Do fluorescent pigments in corals provide photoprotection to their algal endosymbionts and reduce the severity of bleaching? Australian Coral Reef Society Conference.
- Salih, A., Hoegh-Guldberg O & Cox G., 1997. Bleaching responses of corals: the effects of light and elevated temperature on their morphology and physiology. Australian Coral Reef Society Conference.
- Salih, A., Hoegh-Guldberg O & Cox G., 1997. Photoprotection of symbionts by fluorescent sunscreens in reef corals. Australian Coral Reef Society 75th Anniversary Conference.
- Schlichter, D., W. Weber and H.W. Fricke, 1985. A chromatophore system in the hermatypic, deep water coral Leptoseris fragilis (Anthozoa: Hexacorallia). Mar. Biol. 89:143-147.
- Schlichter, D., H.W. Fricke and W. Weber, 1986. Light harvesting by wavelength transformation in a symbiotic coral of the Red Sea twilight zone. Mar. Biol., 91:403-407.
- Schonwald, H., Y. Achituv, and Z. Dubinsky, 1987. Differences in the symbiotic interrelation in dark and light coloured colonies of the hydrocoral Millepora dichotoma. Symbiosis 4:171-184.
- Shagin, D., E. Barsova, Y. Yanushevich, A. Fradkov, K. Lukyanov, Y. Labas, T. Semenova, J. Ugalde, A. Meyers, J. Nunez, E. Widder, S. Lukyanov and M. Matz, 2004. GFP-like proteins as ubiquitous metazoan superfamily: Evolution of functional features and structural complexity. Mol. Biol. Evol., 21(5):841-850.
- Shibata, K., 1969. Pigments and a UV-absorbing substance in corals and a blue-green alga living in the Great Barrier Reef. Plant and Cell Physiol., 10: 325-335.
- Shimomura, O., F. Johnson and Y. Saiga, 1962. J. Cell Comp. Physiol. 59:223-239.
- Shkrob, M., Y. Yanushevich, D. Chudakov, N. Gurskaya, Y. Labas, S. Poponov, N. Mudrik, S. Lukyanov and K. Lukyanov, 2005. Far-red fluorescent proteins evolved from a blue chromoprotein from Actinia equina. Biochem. J., On-line publication.
- Sole-Cava, L.M., and J.P. Thorpe, 1992. Genetic divergence between the color morphs in populations of the common intertidal sea anemones Actinia equina and A. prasina (Anthozoa: Actiniaria) in the Isle of Man. Mar. Biol. 112:243-252.
- Sprung, J. and J. Delbeek, 1997. The Reef Aquarium – A Comprehensive Guide to the Identification and Care of Tropical Marine Invertebrates, Vol. 2. Ricordea Publishing, Coconut Grove, Florida. 546 pp.
- Takabayashi, M. and O. Hoegh-Guldberg, 1995. Ecological and physiological differences between the two colour morphs of the coral Pocillopora damicornis. Mar. Biol., 123: 705-714.
- Takabayashi, M., D. Carter, J. Lopez and O. Hoegh-Guldberg, 2003. Genetic variation of the scleractinian coral Stylophora pistillata, from western Pacific reefs. Coral Reefs 22(1): 17-22.
- Terskikh, A., A. Fradkov, A. Zaraisky, A. Kajava and B. Angres, 2002. Analysis of red mutants. Space around the fluorophore accelerates fluorescence development. J. Biol. Chem., 277(10):7633-7636.
- Todd, P., R. Sidle and L. Chou, 2002. Plastic corals from Singapore: 1. Coral Reefs, 21(4):391.
- Todd, P., R. Sidle and L. Chou, 2002a. Plastic corals from Singapore: 2. Coral Reefs, 21(4):407.
- Todd, P., R. Sidle and L. Chou, 2003. Erratum: Plastic corals from Singapore: 1. Coral Reefs, 22(3):306.
- Tsien, R., 1998. The green fluorescent protein. Annu. Rev. Biochem., 67:509-544.
- Tsutsui, H., S. Karasawa, H. Shimizu, N. Nukina and A. Miyawaki, 2005. Semi-rational engineering of a coral fluorescent protein into an efficient highlighter. EMBO Reports. 6:233-238.
- Tu, H., Q. Xiong, S. Zhen, X. Zhong, L. Peng, H. Chen, X. Jiang, W. Liu, W. Yang, J. Wei, M. Dong, W. Wu and A. Xu, 2003. A naturally enhanced green fluorescent protein from magnificent sea anemone (Heteractis magnifica) and its functional analysis. Biochem. Biophys. Res. Commun., 301(4):879-885.
- Tyree, S., 1998. Reef Building Stony Corals: The Natural Physical Environment. A Review of the Research Papers. D.E. Publishing, Murrieta, Ca. 418 pp.
- Verkhusha, V. and K. Lukyanov, 2004. The molecular properties and applications of Anthozoa fluorescent proteins and chromoproteins. Nature Biotechnology, 22(3):289-296.
- Vermeij, M., L. Delvoye, G. Nieuwland and R. Bak, 2002. Patterns in fluorescence over a Caribbean reef slope: The coral genus Madracis. Photosynthetica, 40(3): 423-429.
- Veron, J., 1993. Corals of Australia and the Indo-Pacific. University of Hawaii Press, Honolulu. 644 pp.
- Veron, J., 1995. Corals In Space and Time – The Biogeography and Evolution of the Scleractinia. Cornell University Press, Ithaca, NY. 321 pp.
- Vestheim, H. and S. Kaartvedt, 2006. Plasticity in coloration as an antipredator strategy among zooplankton. Limnol. Oceanogr., 5(14): 1931-1934.
- Wachter, R. and S. Remington, 1999. Sensitivity of the yellow variant of GFP to halides and nitrate. Curr. Biol., 9(17); R627-R630.
- Weber, W. and H. Gras, 1980. Ultrastructure observations on changes in cell shapes in chromatophores of the Sea Urchin Centrostephanus longispinus. Cell Tiss. Res., 206:21-33.
- Weber, W. and M. Daumbach, 1974. Light-sensitivity of isolated pigment cells of the Sea Urchin Centrostephanus longispinus. Cell Tiss. Res., 148:437-440.
- Wiedenmann, J., C. Elke, K-D. Spindler, and W. Funke, 2000. Cracks in the beta-can: Fluorescent proteins from Anemonia sulcata (Anthozoa, Actinaria). Proc. Natl. Acad. Sci. USA, 97(26): 14091-14096.
- Wiedenmann, J., A. Schenk, C. Röcker, A. Girod, K-D. Spindler and G. Nienhaus, 2002. A far-red fluorescent protein with fast maturation and reduced oligomerization tendency from Entacmaea quadricolor (Anthozoa, Actinaria). Proc. Natl. Acad. Sci. USA, 99, 18: 11646-11651.
- Wiedenmann, J., A. Schenk, C. Röcker, A. Girod, K-D. Spindler and G. Nienhaus, 2002a. Correction for: A far-red fluorescent protein with fast maturation and reduced oligomerization tendency from Entacmaea quadricolor (Anthozoa, Actinaria). Proc. Natl. Acad. Sci. USA, 99, 20:14091-14096.
- Wiedenmann, J., S. Ivanchenko, F. Oswald, F. Schmitt, C. Röcker, A. Salih, K-D. Splinder and G. Nienhaus, 2004. EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion. Proc. Natl. Acad. Sci. USA, 101(45):15905-15910.
- Wilmann, P., J. Battad, T. Beddoe, S. Olsen, S. Smith, S. Dove, R. Devenish, J. Rossjohn and M. Prescott, 2005. The 2.0 Å crystal structure of a pocilloporan at pH 3.5: The structural basis for the linkage between color transition and halide binding. Photochem. Photobiol., 82(2):359-66.
- Yampolsky, I., S. Remington, V. Martynov, V. Potapov, S. Lukyanov and K. Lukyanov, 2005. Synthesis and properties of the chromophore of the asFP595 chromoprotein from Anemonia sulcata. Biochem., 44(15):5788-5793.
- Yanushevic, Y., M. Bulina, N. Gurskaya, A. Savitskii and K. Lukyanov, 2002. Key amino acid residues responsible for the color of green and yellow fluorescent proteins from the coral polyp Zoanthus sp. Russ. J. Bioorg. Chem., 28(4): 274-277.
- Yentsch, C. and D. Phinney. CoBOP Coral Reefs: Optical closure of a coral reef submarine light field. On-line publication.
- Yu, J-K, T-H Liao, C. Chen, T-H Liao, L-S Fang and W-S Tsai, 2000. Do color patterns of Pocillopora damicornis reflect zooxanthellae diversity? Coral Reefs, 19(1): 98-99.
- Yu, M-H., A. Glazer, K. Spencer and J. West, 1981. Phycoerythrins of the red alga Callithamnion. Plant Physiol., 68:482-488.
- Zawada, D. and J. Jaffe, 2003. Changes in the fluorescence of the Caribbean coral Montastraea faveolata during heat-induced bleaching. Limnol. Oceanogr., 481(1, part 2):412-425.
- Zimmer, M., 2005. Glowing Genes: A Revolution in Biotechnology. Prometheus Books, Amherst, New York. 222 pp.





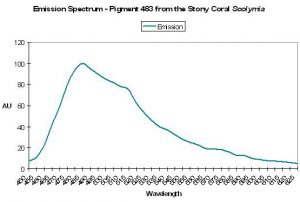
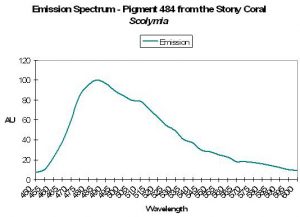

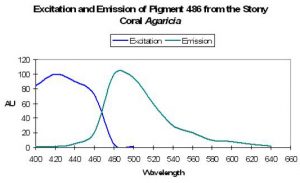

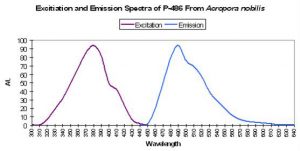
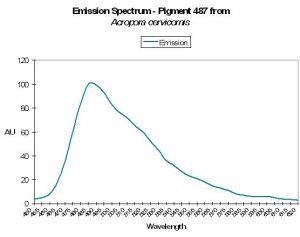

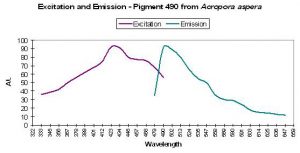


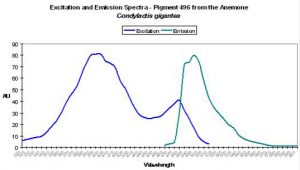
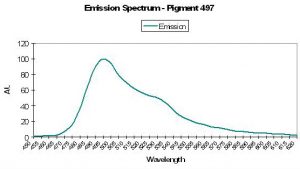
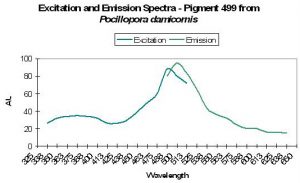
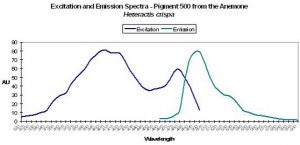
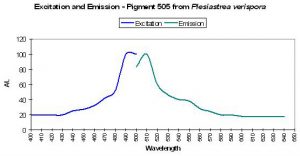
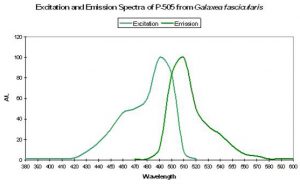

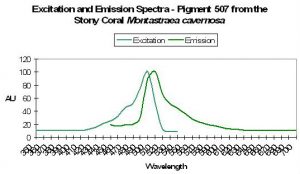
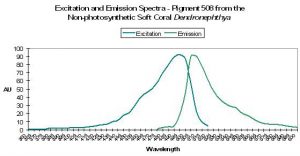
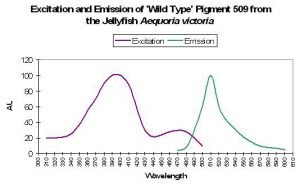
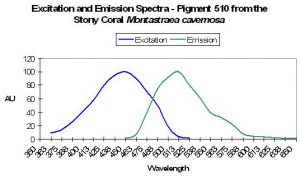

0 Comments