This month our examination of described coral pigments continues with a glimpse of yellow and orange coloration, along with a listing of characteristics associated with photoconvertible fluorescence. It is certain that we do not possess all the answers to pigment photoconversion and other apparent color shifts. However, the results of research projects of many scientists do offer a tantalizing look at how corals’ pigmentation can respond to various environmental stimuli. With this information in hand, we can perhaps begin to predict how corals react to external pressures and manipulate these reactions.
To begin, Table 5 lists the pigments discussed in Part 3 of this series (see Part 1 for a full listing).
| Pigment | Emission | 2 | Excitation | 2 | 3 | Found in | Reference |
|---|---|---|---|---|---|---|---|
| P-573 | 573 | 510 | * | * | * | Ricordea florida | Mazel, 1995 |
| P-574 | 574 | 517 | 506 | 566 | * | Ricordea florida | Labas et al., 2002 |
| P-574 | 574 | 550 | * | * | * | Plesiastrea verispora | Dove et al., 2001 |
| P-575 | 575 | ~630 | ~525 | ~570 | * | Montastraea cavernosa | Mazel, 1997 |
| P-575 | 575 | * | 506 | 555 | * | Montipora (digitata/angulata) | Mazel, unpublished |
| P-575 | 575 | * | 557 | * | * | Dendronephthya | Pakhomov et al., 2004 |
| P-575 | 575 | 630 | 520 | * | * | Scolymia sp. | Mazel et al., 2003 |
| P-575 | 575 | * | ~569 | ~540 | ~500 | Phycoerythrin in coral symbiotic cyanobacteria | Mazel et al., 2004 |
| P-576 | 576 | * | * | * | * | Zoanthus – Mature form of P-522 | Ianushevich et al., 2003 |
| P-580 | 580 | * | * | * | * | Lobophyllia hemprichii (red) | Salih et al., 2004 |
| P-580 | 580 | * | * | * | * | Plesiastrea verispora (blue morph) | Salih et al., 2004 |
| P-580 | 580 | * | * | * | * | Plesiastrea verispora (green morph) | Salih et al., 2004 |
| P-580 | 580 | 520 | 508 | 572 | * | Montastraea cavernosa (mcavRFP) | Labas et al., 2002 |
| P-581 | 581 | * | * | * | * | Lobophyllia hemprichii (red) | Salih et al., 2004 |
| P-582 | 582 | * | 558 | * | * | Trachyphyllia geoffroyi | Ando et al., 2002 |
| P-582 | 582 | 630 | 508 | 572 | * | Montastraea cavernosa (mc1) | Kelmanson & Matz, 2003 |
| P-582 | 582 | * | * | * | * | Goniopora tenuidens | Salih et al., 2004 |
| P-583 | 583 | * | 558 | 530 | 487 | Discosoma sp. | Matz et al., 1999; Baird et al., 2000 |
| P-587-590 | 587-590 | * | * | * | * | Ricordea florida (mouth) | Mazel, 1995 |
| P-587 | 587 | 609 | * | * | * | Madracis pharensis @ 40m (brown tissue) | Vermeij et al., 2002 |
| P-590 | 590 | * | 570 | * | * | Acropora digitifera | Dove et al., 2001 |
| P-593 | 593 | * | 573 | * | * | Discosoma sp. 3 | Labas et al., 2002 |
| P-593 | 593 | * | 405 | * | * | Acropora millepora | Cox and Salih, 2005 |
| P-593 | 593 | * | 558 | * | * | Discosoma sp. 2 | Fradkov et al., 2000 |
| P-593 | 593 | * | 583 | * | * | Favia favus | Tsutsui et al., 2005 |
| P-595 | 595 | * | 574 | * | * | Anemonia sculata | Wiedenmann et al., 2000 |
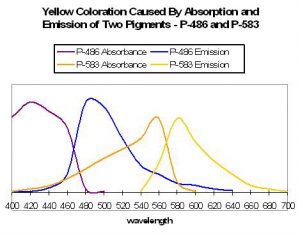
Figure 55. An example of two pigments’ fluorescence resulting in an apparent yellow coloration. After Matz et al., 1999.
Yellow Coloration
Yellow coloration can be due to at least several sets of circumstances. Yellow fluorescence can be the apparent color when two fluorescent pigments – red and green – are present together and in proper proportions. The red fluorescence may be due to either chlorophyll a fluorescence or reddish fluorescent pigments within the coral host tissue. For example, Matz et al. (1999) found a combination of Pigments 486 (green) and 583 (orange-red) appear as yellow (see Figure 55). If the red pigment is in greater proportion than the green pigment, the color of fluoresce can be described as orange in color (see below). Yellow fluorescence can also be attributed to a single pigment or series of pigments resulting in emissions peaking in the yellow portion of the spectrum. Mazel (1997) illustrates this point – he found a yellow pigment in the Elephant-skin coral (Agaricia) with an emission peak in the yellow-green portion of the spectrum (557 nm and two shoulders). Excitation wavelengths peaked at 507 nm (blue-green).
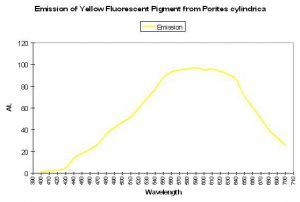
Shibata (1969) reported the absorption spectra of two pigments in a yellow staghorn coral (Acropora species); unfortunately the emission spectra were not reported. See Figure 56. Complementing Shibata’s information is that of Salih et al. (2000) – these researchers report the emission spectrum of a yellow pigment found in Porites cylindrica (see Figure 57). Yellow pigmentation has also been noted in Porites colonies grown in outdoor tanks subjected to intense sunlight (maximum intensity ~1,900 µmol·m2·sec or ~95,000 lux. See Figure 58). Promotion of intense yellow fluorescence has been noted in Acropora specimens when using Iwasaki 400-watt 6,500° K metal halide lamps producing intensities approaching 700 µmol·m2·sec (~35,000 lux).
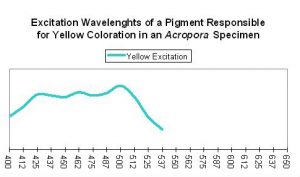
Figure 56. These wavelengths are absorbed by a pigment found within a yellow Acropora specimen. After Shibata, 1969.
In addition, there is a yellow fluorescent pigment called Zoanthus Yellow Fluorescent Protein (or ZoanYFP, as described by, among others, Labas et al., 2002). The peak emission is at 536nm (yellow-green), but extends well past 600nm, giving it a yellow appearance.
P-572 or CP-572
Host: Anemonia sculata
- Excitation: N/A
- Emission: 572nm
- Stokes shift: N/A
- Reference: Verkhusha et al., 2001
- Comments: This pigment is the immature form of the fluorescent pigment P-595 found in Anemonia sculata. Normally considered non-fluorescent, CP-572 is ‘kindled’ to a weakly fluorescent state by exposure to green light.
P-573
Host: Ricordea florida
- Excitation: Not listed
- Emission: 573nm
- Stokes shift: N/A
- Reference: Mazel, 1995
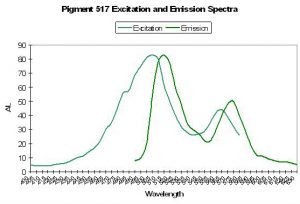
- Comments: Mazel, 1995, reports that the emission of the fluorescent pigment found within Ricordea florida peaks at 573nm, but has a shoulder at 510 nm. These emissions are similar to those found by Labas et al., 2002 in Ricordea florida (rfloFP-517) – the mature form of the green fluorescent protein and is converted a form that has a large emission shoulder at 574. It is converted by light energy, can occur under anaerobic conditions and is not related to chemical oxidation.
Orange Pigments
The visual perception of orange coloration is fairly common in marine invertebrates and for good reason. There are a number of true fluorescent proteins with emissions in the orange portion of the spectrum. Green fluorescence (such as P-496), when in combination with chlorophyll fluorescence and/or perhaps one of the true red fluorescent proteins (P-610, P-620, etc.) accounts for some cases of ‘orange’ fluorescence (Mazel, 1995). Some seem to be truly orange pigments, some of which are listed below, such as that noted in Cynarina lacrymalis (Logan et al., 1990). Mazel (1995, 1997) identified the orange pigment P-575 in the Caribbean Star Coral (Montastrea cavernosa). Blue-green (500 nm) and yellow-green (565 nm) wavelengths are most strongly absorbed. Mazel (unpublished data) also identified a similar pigment in the Orange Velvet Coral (Montipora digitata), with excitation wavelengths at 506 and 555 nm.
Figure 59. Orange fluorescence is visible in this jellyfish and sea-spider. Photograph taken during a night dive off the Kailua-Kona, Hawaii coast by Sara Peck.
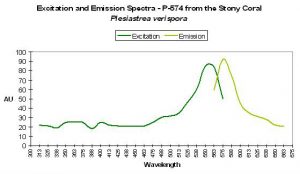
P-574
Host: Plesiastrea verispora
- Excitation: ~560nm
- Emission: 574nm
- Stokes shift: ~14nm
- Reference: Dove et al., 2001
- Comments: Plesiastrea specimens contain many described pigments. See Dove et al., 2001, Gilmore et al., 2003 and Salih et al., 2004. Figure 60 is a chart of P-574.
Host: Ricordea florida
- Excitation: 506nm and 566nm
- Emission: 517nm and 574nm
- Stokes shift: 11nm and 8nm
- Reference: Labas et al., 2002
- Comments: A mature form of P-517?
P-575
Host: Montastrea cavernosa
- Excitation: 500nm and shoulder at 565nm
- Emission: 575nm
- Stokes shift: 10nm/75nm
- Reference: Mazel, 1997
Host: Acropora aspera
- Excitation: 500nm, with a shoulder at 475nm
- Emission: 476nm, with shoulders 510nm and 575nm
- Stokes shift: Unsure
- Reference: Papina et al., 2002, designated this 575nm emission as ‘Orange Band I.’
Host: Acropora aspera
- Excitation: 501nm, with a shoulder at 475nm
- Emission: 478nm, with shoulders 510nm and 575nm
- Stokes shift: Unsure
- Reference: Papina et al., 2002, designated this 575nm emission as ‘Orange Band II.’
Host: Scolymia sp.
- Excitation: 520nm
- Emission: 575nm
- Stokes shift: 55nm
- Reference: Mazel et al., 2003
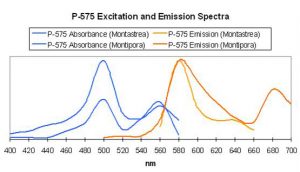
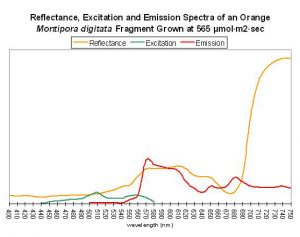
Figure 62. After Mazel, 1997 and unpublished data. Montipora digitata fluorescence at ~680nm in this figure is due to chlorophyll content. Again, this Montipora was tank-raised.
Host: Montipora digitata
- Excitation: ~510nm
- Emission: 575nm
- Stokes shift: 65nm
- Reference: Mazel, unpublished data
- Comments: This Montipora digitata (See Figures 61 and 62) was grown in an aquarium using a 6500K Iwasaki metal halide lamp (providing light intensity of 565 µmol·m²·sec – ~28,000 lux – and a photoperiod of ~12 hours) at the now defunct Aquatic Wildlife Company in Cleveland, Tennessee. A shaded area of the coral (brown in color) was also analyzed for reflectance (reflectance is the ratio of reflected light and down-dwelling light, and will be discussed in detail later in this series).
P-576: Mature Form of P-522
Host: Zoanthus, Pigment 2
- Excitation: 552nm
- Emission: 576nm
- Stokes shift: 24nm
- Reference: Labas et al., 2002
- Comments: This pigment is fluorescent green at first and then completely matures into red fluorescence. See Figure 63.
P-580: Mature Form of Pigment 519
Host: Montastrea cavernosa
- Excitation: 508nm, with a shoulder at 572nm
- Emission: 580nm (mature form)
- Stokes shift: 12nm; 8nm
- Reference: Labas et al., 2002
- Comments: Initially green, this pigment fails to completely mature to red after 60 hours of incubation time in the Russians’ experiments. The green-to-red conversion is mediated by light and is not due to chemical oxidation. The emission is similar to that of P-517 in that it is fairly narrow and symmetrical. Interestingly, excitation wavelengths do not significantly change once the protein has matured from green to red suggesting (but not confirming) fluorescent resonance energy transfer (FRET). There is a distinct shoulder in the emission at ~630nm.
P-581: Mature Form of Pigment 508 (Commercially available as Dendra.)
Host: Dendronephthya sp.
- Excitation: N/A

Figure 65. When green and red fluorescent pigments are found together in the proper proportions, orange fluorescence can be the result. The red pigment could be in chlorophyll a or any other pigment fluorescing red light.
- Emission: 581nm (mature form)
- Stokes shift: N/A
- Reference: Labas, 2002; Lukyanov, 2006.
- Comments: Matures from green to red under intense blue light. There is an apparent yellow intermediate due to color mixing during the transformation from green to red.
P-581: Mature Form of Pigment 516 (Commercially available as EosFP.)
Host: Lobophyllia hemprichii
- Excitation:
- Emission: 581nm (mature form)
- Stokes shift:
- Reference: Nienhaus et al., 2005
- Comments: The ‘Eos’ in EosFP isn’t a synonym for any coral – Eos is the goddess of dawn in Greek mythology. While eloquent, the name is not sufficiently descriptive. My first impression was that Eos stood for Eusmillia, or perhaps Euphyllia, oswaldii (or some other fanciful species). It is also found in the non-photosynthetic soft coral Dendronephthya. So, a protein from E. oswaldii (ha!) is found in a soft coral too? It soon becomes clear why I prefer identifying fluorescent pigments by their emission maxima.
Relative brightness of this red form is 0.68 (when compared to a modified form of A. victoria GFP).
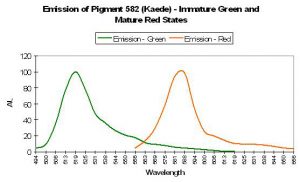
P-582: Mature Form of P-518 (Commercially available as Kaede.)
Host: Trachyphyllia geoffroyi
- Excitation: 572nm (mature form)
- Emission: 582nm (mature form)
- Stokes shift: 10nm
- Reference: Ando et al., 2002.
- Comments: Pigment-582 was isolated from an ‘Open Brain coral’ (Trachyphyllia geoffroyi) by Ando et al., 2002. Interestingly, this pigment matures from a green fluorescent protein to an orange/red one when exposed to strong ultraviolet radiation (1,820 µW/cm² @ 365 nanometers) or violet light at ~400 nm (conversion can occur under anaerobic conditions but not in darkness – it is driven by exposure to light and is not due to chemical oxidation). Kaede is Japanese for ‘maple leaf’ – a reference to the leaf’s seasonal color change from green to red. Relative brightness of P-518 (the immature green form is 2.59 (when compared to a modified form of A. victoria GFP); the relative brightness of the red form (P-582) is 0.59.
Discovery of the photoconvertible nature of P-582 was discovered when a vial of P-518 was inadvertently left on a laboratory bench and exposed to light. (I have tried for years to convince my supervisor that a small indifference to laboratory housekeeping can lead to great discoveries. After all, the antibiotic penicillin was found growing on an old, moldy sandwich. My boss remains unconvinced.)
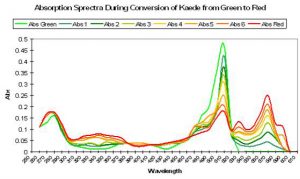
Ando and his team bought their Trachyphyllia at a local fish store. While there, they noted, but did not buy, fluorescent yellow Open Brain corals. It is tempting to think that these yellow animals’ GFPs were in transition from green to red, with yellow being the perceived color when red and green granules are present in correct proportions. This seems to be confirmed by the work of Dittrich et al. (2005); they found no intermediate fluorescent states (such as yellow) when green pigments transform to red ones.
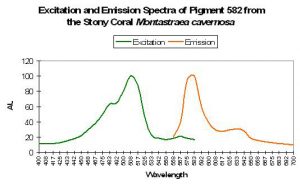
Host: Montastraea cavernosa
- Excitation: 508nm, with a shoulder at 572nm
- Emission: 582nm, with a shoulder at 630nm
- Stokes shift: 80nm/58nm
- Reference: Kelmanson and Matz, 2003.
- Comments: This pigment was found in both red and green M. cavernosa colonies collected in the Florida Keys National Marine Sanctuary. Excitation, emission and Stokes shift are not similar to Kaede.
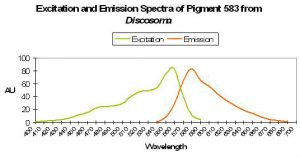
P-583: Mature Form of Pigment 500 (Commercially available as DsRed.)
Host: Discosoma 1 sp.
- Excitation: 558nm
- Emission: 583nm
- Stokes shift: 25nm
- Reference: Matz et al., 1999; Labas et al., 2002; Gross et al., 2000.
- Comments: Initially green, this protein matures (albeit slowly and incompletely) to a red form via chemical oxidation. Fluorescent quantum yield is reported to be 0.23, and relative brightness (A. victoria GFP standard) is 0.24 (Matz et al., 1999). The red fluorescence is often seen in the whole polyp, but is sometimes concentrated in spots on the oral disk. Substitution of only one amino acid in the protein prevents maturation of green to red (Baird et al., 2000).
P-587-590
Host: Ricordea florida
- Excitation: Not listed
- Emission: 587-590nm
- Stokes shift: N/A
- Reference: Mazel, 1995
- Comments: Found only in Ricordea florida and then only around the mouth.
P-590
Host: Acropora digitifera
- Excitation: 570nm
- Emission: 590nm
- Stokes shift: 20nm
- Reference: Dove et al., 2001
- Comments: This particular coral was purple-blue (suggesting the presence of a non-fluorescent chromoprotein); maximum tissue absorbance is 578nm. This fact that the absorbance is identical to that seen in the anemone Heteractis crispa, suggests that this coral contains the same pigment.
P-593
Host: Discosoma sp., Pigment 2
- Excitation: 487nm, 530nm, and 558nm
- Emission: 593nm
- Stokes shift: ~35nm
- Reference: Fradkov et al., 2000; Garcia-Parajo et al., 2001
Host: Discosoma sp., Pigment 3
- Excitation: 573nm
- Emission: 593nm
- Stokes shift: 20nm
- Reference: Labas et al., 2002
- Comments: The excitation wavelengths are different from those seen in Discosoma, Pigment 2 (above), although the emission is much the same.
Host: Acropora millepora
- Excitation: 405nm
- Emission: 593nm
- Stokes shift: 187nm
- Reference: Cox and Salih, 2005.
- Comments: Excitation via pulsed 405nm laser.
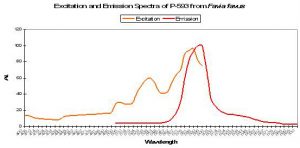
P-593: Mature Form of Pigment 517
Host: Favia favus
- Excitation: 583nm
- Emission: 593nm
- Stokes shift: 12nm
- References: Tsutsui et al., 2005.
- Comments: Initially green, this pigment fails to completely mature to red after 60 hours of incubation time in the Russians’ experiments. The emission is similar to that of P-517 (see Figure 73) in that it is fairly narrow and symmetrical. Interestingly, excitation wavelengths do not significantly change once the protein has matured from green to red suggesting (but not confirming) Förster resonance energy transfer (FRET). Relative brightness (compared to an engineered GFP from Aequorea victoria) is 0.68.
P-595: Mature Form of Pigment 562
Host: Anemonia sculata
- Excitation: 575nm
- Emission: 595nm
- Stokes shift: 20nm
- References: Wiedenmann et al., 2002; Wiedenmann et al., 2002a; Lukyanov et al., 2000; Andresen et al., 2005.
- Comments: This pigment is similar to P-562 in that it is found in Anemonia sulcata var. vulgaris (Wiedenmann et al., 2002a) and demonstrates fluorescence only to instrumentation and not the unaided eye (the fluorescent quantum yield is <0.001). It is generally regarded as a non-fluorescent chromoprotein (Wiedenmann et al., 2002). However, Andresen et al., 2005 report that ‘wild type’ P-595 can be converted (‘kindled’) to its barely fluorescent state with exposure to green light. The switch is reversible and can be promptly converted to its non-fluorescent state by exposure to blue light. Even without blue light, P-595 reverts back eventually to its state that is incapable of fluorescence.
Phycoerythrin
Phycoerythrin, a biliprotein, is a photosynthetic accessory or antenna pigment found in red algae (including calcareous forms) and cyanobacteria. Hobbyists tend to lump cyanobacteria (sometimes referred to as ‘slime algae’) into a category akin to an ‘axis of evil’ but this shouldn’t necessarily be so. Mazel et al., 2004 found unicellular cyanobacteria existing within the tissues of the stony coral Montastraea cavernosa. These nitrogen-fixing bacteria convert dissolved nitrogen gas to nitrogenous compounds that are available for use by symbiotic zooxanthellae, thus potentially giving these corals a competitive edge in a nitrogen-poor environment. In return, it is thought that zooxanthellae provide cyanobacteria with organic substances such as glycerol for use as food. The discovery of this symbiosis is important enough but is perhaps of most interest to hobbyists since phycoerythrin is a fluorescent pigment, with an emission peaking at 573-580nm (Mazel, 1995). Cyanophytes have also been found in symbiosis with sponges and other marine animals (Larkum et al., 1987).
Figure 76 shows the absorption spectrum of phycoerythrin with peaks at ~497nm, 539nm and 565nm. Variations in the concentrations of two bilin groups (phycoerythrobilin and phycourobilin) can alter the absorption spectra, and the 539nm absorption is sometimes absent in certain red algae. Interestingly, the ratio of phycoerythrobilin and phycourobilin in phycoerythrin can be manipulated by varying the light intensity during the growth of the red alga Callithamnion roseum (Yu et al., 1981). Spectral quality may also play a role in determining apparent coloration. Laboratory studies have shown that light enhanced in the green-yellow portion (500-575nm) encourages biosynthesis of phycoerythrin, while red light (600-700nm) inhibits production and hence accumulation of this pigment. This should not necessarily be regarded as proof of chromatic adaptation since Kirk (2000) states that increased concentrations of phycoerythrin can occur in response to lessened light intensity (there is some evidence that same adaptation occurs in zooxanthellae – see Kinzie and Hunter, 1987). Light intensity – PAR – may also play an important part in husbandry of phycoerythrin-containing corals. Moore and Chisolm (1999) suggest that intensity exceeding 250 µmol·m²·sec will inhibit growth of the high light adapted cyanobacterium Prochlorococcus. PAR of ~125 µmol·m²·sec was harmful to the low light-adapted growths of Prochlorococcus. Aside from suggesting ‘strong light’ will control cyanobacterial outbreaks within aquaria (another anecdotal story among hobbyists), it also seems to suggest that corals containing phycoerythrin ‘require’ relatively little light.

Figure 76. Spectral qualities of the accessory pigment phycoerythrin (found in some coralline alga as well as cyanobacteria sometimes in symbioses with corals). After Cyanotech specification sheet.
P-595 – Mature Form of P-583
Host: Discosoma sp.
- Excitation: Unknown
- Emission: 595nm
- Stokes shift: Unknown
- Reference: Cotlet et al., 2001.
- Comments: Conversion of this pigment from an emission of 583nm (orange-red) to 595nm (red) results from exposure of yellow light with a peak at ~ 570nm.
Photoconversion
Photoconversion of some brown corals to colorful ones (or vice versa) are phenomena that can confound any hobbyist (or researcher, diver, etc., for that matter). The ability to shift coloration seems to be a genetic trait limited to a minority of coral species.
| From | To | Host | Protein | Activator | Direction | Shift | 2 Peak Ex / Em | Reference |
|---|---|---|---|---|---|---|---|---|
| P-484 | 515 | Acropora secale | Double-peaked excitation suggests photoconversion is possible | Blue – green to Green? | Yes | Papina et al., 2002 | ||
| P-496 | ? | Condylactis gigantea | Double-peaked excitation suggests photoconversion is possible | ? | Yes | Labas et al., 2002 | ||
| P-499 | P-522 | Anemonia sculata | ? | Green – blue to Green? | * | Wiedenmann, 2000 | ||
| P-500 | 583 | Discosoma | DsRed | Exposure to light at ~ 488nm | ? | Green – blue to Orange | * | Gross et al., 2000; Cotlet et al., 2001 |
| P-500 | Heteractis crispa | Double-peaked excitation suggests photoconversion is possible | ? | Yes | Labas et al., 2002 | |||
| P-505 | 508 / 572 | Montastraea cavernosa | Double-peaked emission, peak at 508nm, with shoulder @ 572nm | Green – blue to Orange | Yes | Labas et al., 2002 | ||
| P-505 | 506 / 566 | Ricordea florida | Double-peaked emission, peak at 506nm, with shoulder @ 566 nm | Green – blue to Orange | Yes | Labas et al., 2002 | ||
| P-506 | 538 | Zoanthus sp. | Site-directed mutagenesis – 3 amino acid substitutions – not observed in wild | Green to Yellow | ? | Gurskaya et al., 2002 | ||
| P-508 | 575 | Dendronephthya sp. | Dendra | Blue Light @ 488nm | ? | Green to Red | * | Gurskaya et al., 2006 |
| P-508 | 575 | Dendronephthya sp. | DendFP | UV-A at 366nm | Irreversible | Green to Red | * | Pakhomov et al., 2004 |
| None | 509 | Aequorea victoria | GFP | Matures only with oxygen present | Irreversible | None to Green | * | Tsien, 1998 |
| P-509 | 600 | Aequorea victoria | GFP | Anoxic / anaerobic Conditions / Blue light | ? | Green to Red | * | Tsien, 1998; Elowitz et al., 1997 |
| ~516 | 582 | Montastraea cavernosa | mcav | Depth – light – related? | ? | Green to Orange | Yes | Kelmanson & Matz, 2003; Todd et al., 2000 |
| P-516 | 581 | Lobophyllia hemprichii | Eos | UV @ 390nm / Violet Light ~400nm | ? | Green to Red | * | Nienhaus et al., 2005 |
| P-517 | 574 | Ricordea florida | rfloRFP | UV / Violet Light | ? | Green to Red | * | Labas et al., 2002 |
| P-517 | 580 | Montastraea annularis | * | UV / Violet Light | ? | Green to Red | * | Shagin et al., 2004 |
| P-517 | 593 | Favia favus | Kikume | UV & Violet (350 – 420 nm) | ? | Green to Red | * | Tsutsui et al., 2005 |
| P-518 | None | “Pectiniidae” | Dronpa | ~490 nm to bleach | Reversible | Green to None | * | Ando et al., 2004 |
| P-518 | 582 | Trachyphyllia geoffroyi | Kaede / tgeoRFP | UV – Violet Light (350 – 410 nm) | Irreversible | Green to Red | * | Ando et al., 2002; Dittrich et al., 2005 |
| P-519 | 580 | Montastraea cavernosa | mcavRFP | UV – Violet Light | Irreversible | Green to Red | Yes | Labas et al., 2002 |
| P-520 | 611 | Entacmaea quadricolor | equaRFP | Chemical Oxidation | ? | Green to Red | Yes | Nienhaus et al., 2003; 2005 |
| P-522 | 576 | Zoanthus sp. | zoan2RFP | ? | ? | Green to Orange | * | Labas et al., 2002; Ianushevich et al., 2003 |
| P-538 | ? | Zoanthus sp. | zoanYFP | Double peaked excitation | ? | ? | Yes | Labas et al., 2002 |
| CP-562 | FP-595 | Anemonia sculata | “Kindling” Protein | Green Light (540 – 560 nm) | Reversible / Irreversible | None to Red | * | Verkhusha et al., 2001; Chudakov et al., 2003 |
| CP-580 | FP-436 | Goniopora tenuidens | gtCP | Chemical Oxidation | Reversible / Irreversible | Red to Green | * | Martynov et al., 2003 |
| P-582 | P-630 | Montastraea cavernosa | mc1 or mcav1 | ? | ? | Orange to Red? | Yes | Kelmanson and Matz, 2003 |
| P-583 | P-595 | Discosoma | DsRed | Exposure to light at ~570 nm | ? | Red to Super Red | * | Cotlet et al., 2001 |
| P-520 | 611 | Entacamea quadricolor | equaRFP | Chemical Oxidation | ? | Green to Red | * | Nienhaus et al., 2005 |
Comments on Photoconversion
Photoconversion of fluorescent pigments is often associated with the presence of ultraviolet radiation and visible light in the violet/blue bandwidths. It may come as a surprise that wavelengths that do not promote photosynthesis to a great degree (“junk” wavelengths, photosynthetically speaking) can be responsible for color shifts. These ‘junk wavelengths’ include shortwave green light and yellow light at ~570nm.

Figure 77. Wavelengths and bandwidths known to induce coloration changes in anthozoans. The bottom row of numbers represents wavelengths. The vertical spikes and horizontal bands above the spectrum represent important specific wavelengths and bandwidths. See Table 6 for details.
Ultraviolet energy has long had an anecdotal link to inducing vivid coloration in many coral species, and information in Table 6 suggests that UV is necessary for colorful corals. Is UV really necessary for coloration?
Next time, our discussion continues a look at lamp spectra, transmission qualities of various ‘UV shields,’ and how they probably relate to coral color. We’ll also scrutinize various reasons for red fluorescence. It will also include a brief review of scant evidence linking nutrients and ‘supplements’ to coral coloration.
See Part 1 for a full listing of references. Anyone wishing to correspond with the author via email may do so at [email protected].









0 Comments